ISSUE1409
Two new trivalent influenza vaccines, Flucelvax (Novartis) and Flublok (Protein Sciences), will soon be available for prevention of seasonal influenza in adults ≥18 years old (Flucelvax) and 18-49 years old (Flublok). Unlike other available influenza vaccines, neither vaccine is produced in eggs, removing any concern regarding use in egg-allergic patients. Avoiding the use of eggs should allow for faster production of these 2 new vaccines, which could be especially beneficial during a pandemic.
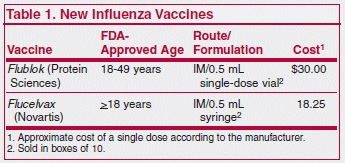
Flucelvax is prepared in a similar manner to other influenza vaccines, but the influenza virus is grown in canine kidney cell culture instead of chicken eggs. In an unpublished study summarized in the package insert comparing Flucelvax with placebo in more than 11,000 patients during the 2007-2008 season, the new vaccine was effective in preventing about 84% of cases of influenza due to matching strains and was about 70% effective when non-matching strains were included.
Flublok is produced without use of influenza virus or chicken eggs; a gene that encodes for hemagglutinin antigen (HA) is introduced into baculovirus, a virus that infects insect cells, and the replicating baculovirus produces large amounts of HA. In a study in about 4,600 adults conducted during one influenza season, Flublok was about 45% effective compared to placebo in preventing culture-confirmed influenza despite a significant antigenic mismatch between the vaccine and circulating viruses (96% of circulating strains did not match the vaccine). It was 75% effective in preventing illness caused by matching strains.1
Both Flublok and Flucelvax provide protection against the same strains as the other vaccines available for the 2012-2013 influenza season.2
