ISSUE1621
- Mark Abramowicz, M.D., President: no disclosure or potential conflict of interest to report
- Jean-Marie Pflomm, Pharm.D., Editor in Chief: no disclosure or potential conflict of interest to report
- Brinda M. Shah, Pharm.D., Consulting Editor: no disclosure or potential conflict of interest to report
- Discuss the evidence supporting the use of low-dose colchicine in patients with coronary artery disease.
- Colchicine has anti-inflammatory effects and has been used off-label to treat patients with coronary artery disease.
- In 2 randomized clinical trials, addition of a low dose of colchicine (0.5 mg once/day) to standard therapy reduced the risk of cardiovascular events compared to placebo in patients with a recent myocardial infarction and in those with chronic coronary artery disease.
- Can cause GI adverse effects, typically in first days of treatment.
- Myopathy, blood dyscrasias, and rhabdomyolysis can occur.
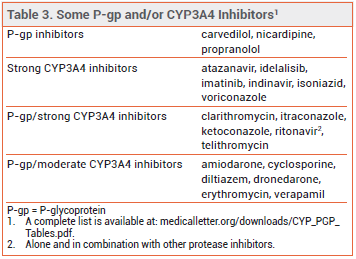
- Increased risk of adverse effects with concomitant use of drugs that inhibit P-glycoprotein (P-gp) and/or CYP3A4.
- Contraindicated in patients with renal or hepatic impairment taking P-gp or strong CYP3A4 inhibitors.
Outline
Tables |
The centuries-old anti-inflammatory drug colchicine (Colcrys, and others) is FDA-approved for prophylaxis and treatment of gout flares and for treatment of familial Mediterranean fever. It is also used off-label to treat pericarditis and pericardial pain after ablation for atrial fibrillation, and in recent years has been investigated for reduction of cardiovascular risk in patients with coronary artery disease (CAD).
MECHANISM OF ACTION — Inflammation is now thought to play an important role in atherosclerosis and the progression of coronary artery disease.1 Colchicine interferes with the activation of interleukin 1-β, inhibits β-tubulin polymerization, and prevents the activation and migration of neutrophils and their interaction with platelets.
CLINICAL STUDIES — ACS – A randomized, double-blind trial (COLCOT) compared a low dose of colchicine (0.5 mg once daily) to placebo, both added to standard therapy, in 4745 patients who had had a myocardial infarction within 30 days before enrollment. After a median follow-up of 22.6 months, a composite of cardiovascular death, resuscitated cardiac arrest, myocardial infarction, stroke, or urgent hospitalization for angina leading to coronary revascularization, the primary endpoint, occurred in 5.5% of patients treated with colchicine versus 7.1% of those who received placebo (HR 0.77; 95% CI 0.61 to 0.96; p=0.02).2
In a second randomized, double-blind trial (Australian COPS), 795 patients presenting with acute coronary syndrome (ACS) and evidence of CAD on coronary angiography were treated with a higher dose of colchicine (0.5 mg twice daily for the first month and once daily thereafter) or placebo and followed for at least 12 months. The primary endpoint was a composite of all-cause mortality, acute coronary syndrome, ischemia-driven urgent revascularization, and noncardioembolic ischemic stroke. At 365 days, a primary endpoint event had occurred in 24 patients treated with colchicine and in 38 who received placebo (HR 0.65; 95% CI 0.38-1.09; p=0.09). In a post hoc analysis of the composite endpoint substituting cardiovascular death for total death, the reduction in events with colchicine was statistically significant compared to placebo (5% vs 9.5%; HR 0.51; 95% CI 0.29-0.89; p=0.019).3
Stable CAD – In a randomized, double-blind trial (LoDoCo2), colchicine 0.5 mg once daily or placebo was added to standard therapy in 5522 patients with chronic coronary artery disease, as evidenced by coronary angiography, computed tomography angiography, or a coronary artery calcium score (Agatston score >400). Most patients (84.4%) had a history of acute coronary syndrome, but they were required to have been clinically stable for at least 6 months before enrollment. After a median follow-up of 28.6 months, a composite of cardiovascular death, nonprocedural myocardial infarction, ischemic stroke, or ischemia-driven coronary revascularization, the primary endpoint, occurred in 6.8% of colchicine-treated patients versus 9.2% of those who received placebo (HR 0.69; 95% CI 0.57 to 0.83; p<0.001).4
ADVERSE EFFECTS — GI adverse effects, including diarrhea, nausea, vomiting, and abdominal pain, are the most common effects of colchicine. These symptoms typically occur within days of starting treatment and are usually mild and transient. Myopathy has been reported; the risk may be increased with concomitant use of statins or other lipid-lowering drugs. Life-threatening toxicities, including blood dyscrasias and rhabdomyolysis, have been reported with therapeutic doses of colchicine. Patients with hepatic or renal impairment are at increased risk for adverse effects. Severe renal (CrCl <30 mL/min) and hepatic (Child-Pugh C) impairment have been associated with colchicine toxicity; dosage reductions may be required.
In all three clinical trials, the incidence of non-cardiovascular death was numerically higher in colchicine-treated patients; the differences between colchicine and placebo were not statistically significant, except in the Australian COPS trial (5 vs 0; p=0.024); 4 of the 5 deaths were from infection and 3 of the patients who died had discontinued colchicine during the first month of the trial.
PREGNANCY AND LACTATION — Although there are no adequate studies of the effects of colchicine in human pregnancy, observational data over several decades suggest that colchicine doses up to 2 mg daily are not associated with an increased risk for major birth defects, miscarriage, or adverse maternal or fetal outcomes. Colchicine is secreted into human breast milk; according to the label, no adverse effects were reported in 187 breastfed infants exposed to the drug.
DRUG INTERACTIONS — Colchicine is a P-glycoprotein (P-gp) and CYP3A4 substrate. Concomitant use of P-gp and/or CYP3A4 inhibitors increases colchicine serum concentrations and the risk of adverse effects (see Table 3); the dosage of colchicine may need to be reduced. The drug is contraindicated in patients with renal or hepatic impairment who are taking P-gp or strong CYP3A4 inhibitors.5
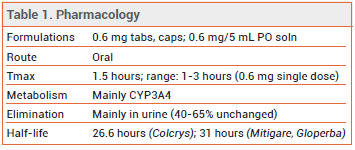
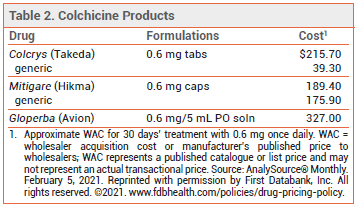
DOSAGE — The 0.5-mg formulation of colchicine used in the CAD clinical trials is not available in the US (see Table 2). The FDA-approved dosage ranges from 0.6 mg once or twice daily for prophylaxis of gout flares to 1.2-2.4 mg daily in one or two doses for treatment of familial Mediterranean fever.
CONCLUSION — Adding a low dose of colchicine (0.5 mg once daily) to standard therapy reduced cardiovascular risk in patients with coronary artery disease in two clinical trials and in a post hoc analysis from a third trial. Colchicine could be considered for inclusion in the regimens used to treat such patients.
- P Libby. Inflammation in atherosclerosis—no longer a theory. Clin Chemistry 2021; 67:131.
- J-C Tardif et al. Efficacy and safety of low-dose colchicine after myocardial infarction. N Engl J Med 2019; 381:2497.
- SM Nidorf et al. Colchicine in patients with chronic coronary disease. N Engl J Med 2020; 383:1838.
- DC Tong et al. Colchicine in patients with acute coronary syndrome: the Australian COPS randomized clinical trial. Circulation 2020; 142:1890.
- Inhibitors and inducers of CYP enzymes and P-glycoprotein. Med Lett Drugs Ther 2020 Sep 10 (epub). Available at: http://secure.medicalletter.org/downloads/CYP_PGP_Tables.pdf.