ISSUE1635
- Mark Abramowicz, M.D., President: no disclosure or potential conflict of interest to report
- Jean-Marie Pflomm, Pharm.D., Editor in Chief: no disclosure or potential conflict of interest to report
- Brinda M. Shah, Pharm.D., Consulting Editor: no disclosure or potential conflict of interest to report
- Michael Viscusi, Pharm.D., Associate Editor: no disclosure or potential conflict of interest to report
- Discuss the potential benefi ts and timing of a booster dose of the Pfizer/BioNTech COVID-19 vaccine (Comirnaty) and list those persons for whom a booster dose should be considered.
On September 22, on the advice of its Vaccines and Related Biologic Products Advisory Committee, the FDA expanded the Emergency Use Authorization (EUA) for the Pfizer/BioNTech mRNA-based COVID-19 vaccine (Comirnaty) to include administration of a booster dose ≥6 months after a 2-dose primary series in adults who are ≥65 years old or at high risk for severe COVID-19 because of an underlying medical condition or frequent institutional or occupational exposure to SARS-CoV-2 (see Table 1).1 The FDA Advisory Committee recommended against authorization of a booster dose of Comirnaty for all persons ≥16 years old, citing a lack of adequate data.
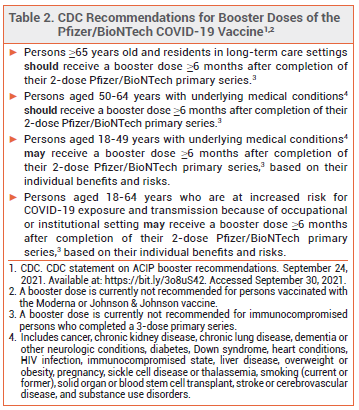
ACIP — The Advisory Committee on Immunization Practices (ACIP) recommended to the CDC that booster doses of Comirnaty be administered to persons who are ≥65 years old, adult residents of long-term care facilities, and persons aged 50-64 years who have an underlying medical condition. The ACIP also recommended that booster doses be considered for younger adults with underlying medical conditions, but advised against their use on the basis of institutional or occupational exposure to SARS-CoV-2.
CDC — The CDC, which regulates US public vaccination programs, accepted most of the ACIP's recommendations, but stated that booster doses may indeed be considered for adults 18-64 years old who are at increased risk for severe COVID-19 because of frequent institutional or occupational exposure to SARS-CoV-2 (see Table 2).2
DOSAGE AND ADMINISTRATION — The booster dose of Comirnaty is the same as the dose for primary immunization (30 mcg IM). A single booster dose may be given ≥6 months after completion of a 2-dose primary series.3
CLINICAL STUDIES — Expansion of the EUA to include booster doses was based on cohort data from the US and Israel and on the results of an immunogenicity study.
Waning Immunity – In a retrospective cohort study of ~3.4 million persons ≥12 years old in the US, those who received two doses of Comirnaty were significantly less likely to be infected with SARS-CoV-2 than those who were not vaccinated, but the relative risk reduction associated with vaccination declined from 88% at ≤1 month to 47% at ≥5 months after the second dose. Vaccination was also associated with a lower risk of hospitalization due to COVID-19; the relative risk reduction did not change significantly over time (87% at ≤1 month; 88% at ≥5 months).4
In a study that examined positive PCR test results for SARS-CoV-2 infection in Israel over 3 weeks in July 2021, adults ≥60 years old who completed a 2-dose primary series of the Pfizer/BioNTech vaccine in the second half of January 2021 had a significantly higher rate of infection than those who completed their series in the second half of March 2021 (3.2 vs 1.6 cases/1000 persons). Similarly, adults ≥60 years old who completed their series in January had a significantly higher rate of severe COVID-19 than those who completed it in March (0.29 vs 0.15 cases/1000 persons).5
Booster Immunogenicity – In an unpublished longitudinal immunogenicity study (summarized in the FDA Fact Sheet), 210 adults 18-55 years old who had completed a 2-dose primary series of Comirnaty about 6 months previously and had no evidence of prior SARS-CoV-2 infection were given a booster dose. Geometric mean titers of anti-SARS-CoV-2 neutralizing antibodies 1 month after the booster dose were 3.29-fold higher than they were 1 month after the second primary-series dose.3
Booster Efficacy – In a one-month cohort study in ~1.1 million Israeli residents who had completed a 2-dose primary series of the Pfizer/BioNTech vaccine ≥5 months previously, persons who received a booster dose had significantly lower rates of SARS-CoV-2 infection (by 11.3-fold) and severe COVID-19 (by 19.5-fold) beginning 12 days after administration compared to those who did not.6
ADVERSE EFFECTS — Adverse effects with a third dose of an mRNA-based COVID-19 vaccine appear to be similar to those with the second primary-series dose.7 In the immunogenicity study in adults 18-55 years old, lymphadenopathy occurred more commonly with the booster dose of the Pfizer/BioNTech vaccine than with primary-series doses (5.2% vs 0.4%), but no cases of hypersensitivity reactions, Bell’s palsy, or myocarditis/pericarditis were reported in booster dose recipients.3,8
OTHER VACCINES — Booster doses of the mRNA-based COVID-19 vaccine manufactured by Moderna (Spikevax) and the adenovirus-based COVID-19 vaccine manufactured by Johnson & Johnson (Janssen) have not been authorized to date. The FDA and CDC are expected to consider the appropriateness of booster doses for recipients of these vaccines in the near future.
CONCLUSION — The FDA has authorized use of a single booster dose of the Pfizer/BioNTech mRNA-based COVID-19 vaccine (Comirnaty) administered ≥6 months after completion of the 2-dose primary series in certain adults. The efficacy of primary immunization with Comirnaty in preventing SARS-CoV-2 infection appears to decrease over time, especially in older persons, and administration of a booster dose has been associated with decreased rates of infection and severe COVID-19. Whether booster doses of Comirnaty will eventually be recommended for the general population remains to be determined. Booster doses of the Moderna and Johnson & Johnson COVID-19 vaccines are not currently recommended.
- FDA News Release. FDA authorizes booster dose of Pfizer-BioNTech COVID-19 vaccine for certain populations. September 22, 2021. Available at: https://bit.ly/3ilp0B1. Accessed September 30, 2021.
- CDC. CDC statement on ACIP booster recommendations. September 24, 2021. Available at: https://bit.ly/3o8uS42. Accessed September 30, 2021.
- FDA. Fact sheet for health care providers administering vaccine (vaccination providers). Emergency Use Authorization (EUA) of the Pfizer-BioNTech COVID-19 vaccine to prevent coronavirus disease 2019 (COVID-19). September 22, 2021. Available at: https://bit. ly/37fX1NG. Accessed September 30, 2021.
- SY Tartof et al. Six-month effectiveness of BNT162b2 mRNA COVID-19 vaccine in a large US integrated health system: a retrospective cohort study. 2021 August 23 (preprint). Available at: https://bit.ly/3CJwzJ8. Accessed September 30, 2021.
- Y Goldberg et al. Waning immunity of the BNT162b2 vaccine: a nationwide study from Israel. medRxiv 2021 August 30 (preprint). Available at: https://bit.ly/2XXy4Vr. Accessed September 30, 2021.
- YM Bar-On et al. Protection of BNT162b2 vaccine booster against Covid-19 in Israel. N Engl J Med 2021 September 15 (epub).
- AM Hause et al. Safety monitoring of an additional dose of COVID-19 Vaccine — United States, August 12–September 19, 2021. MMWR Morb Mortal Wkly Rep 2021 September 28 (epub).
- FDA. FDA review of effectiveness and safety of COMIRNATY (COVID-19 vaccine, mRNA) booster dose. Biologics License Application supplement. Vaccines and Related Biological Products Advisory Committee. September 17, 2021. Available at: https://bit.ly/2ZhqsgQ. Accessed September 30, 2021.