ISSUE1650
- Mark Abramowicz, M.D., President: no disclosure or potential conflict of interest to report
- Jean-Marie Pflomm, Pharm.D., Editor in Chief: no disclosure or potential conflict of interest to report
- Brinda M. Shah, Pharm.D., Consulting Editor: no disclosure or potential conflict of interest to report
- Review the efficacy and safety of the new higher dose of injectable semaglutide (Ozempic) for treatment of type 2 diabetes.
The FDA has approved a higher-dose injectable formulation of the long-acting glucagon-like peptide-1 (GLP-1) receptor agonist semaglutide (Ozempic) for treatment of type 2 diabetes in adults. A single SC injection of the new 8 mg/3 mL formulation delivers 2 mg of semaglutide.
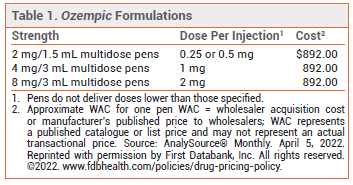
SEMAGLUTIDE FORMULATIONS — Ozempic has been available since 2018 in two multidose pens: one that delivers doses of 0.25 or 0.5 mg and one that delivers 1-mg doses (see Table 1). Up to 30% of patients treated with 1-mg doses of the drug do not achieve the desired A1C goal.1,2 An oral formulation of semaglutide (Rybelsus) has been available for treatment of type 2 diabetes since 2019.3 Injectable semaglutide is also available in a higher dose (2.4 mg) as Wegovy for chronic weight management in adults with or without type 2 diabetes who have a body mass index (BMI) ≥30 kg/m2 or a BMI ≥27 kg/m2 and at least one weight-related comorbidity.4
CLINICAL STUDIES — FDA approval of the new formulation was based on the results of a double-blind trial (SUSTAIN FORTE) in 961 patients with type 2 diabetes that was inadequately controlled on metformin with or without a sulfonylurea. Patients were randomized to receive semaglutide 1 or 2 mg SC once weekly. Patients in the 2-mg group had statistically significantly greater mean changes from baseline at 40 weeks in A1C (-2.2% vs -1.9%) and weight (-6.9 kg vs -6.0 kg) compared to those in the 1-mg group.5
ADVERSE EFFECTS — GI adverse effects (nausea, vomiting, diarrhea, constipation) are common with semaglutide. In SUSTAIN FORTE, GI adverse effects occurred in 34% of patients in the 2-mg group and in 31% of those in the 1-mg group. Acute pancreatitis and cholelithiasis have been reported with use of injectable and oral semaglutide. The labels of all semaglutide formulations include a boxed warning about a risk of thyroid C-cell tumors (based on animal data, no corroborating human data); the drug is contraindicated for use in patients with a personal or family history of medullary thyroid carcinoma and in those with multiple endocrine neoplasia syndrome type 2.
DOSAGE AND ADMINISTRATION — The starting dosage of Ozempic for treatment of type 2 diabetes is 0.25 mg injected SC once weekly in the abdomen, thigh, or upper arm. After 4 weeks, if tolerated, the dose should be increased to 0.5 mg. If additional glycemic control is needed, the dose can be doubled every 4 weeks to a maximum of 2 mg once weekly.
Unused pens should be stored in the refrigerator; after first use, they can be kept at room temperature for up to 56 days. A new needle should be attached for each injection and injection sites should be rotated each week. Ozempic does not need to be reconstituted before administration.
CONCLUSION — A 2-mg dose of the injectable GLP-1 receptor agonist semaglutide (Ozempic) has been approved by the FDA for use in patients with type 2 diabetes that requires additional glycemic control. In one clinical trial, addition of semaglutide 2 mg once weekly to standard treatment reduced A1C and weight more than addition of a 1-mg dose of the drug, but the effect was modest. GI adverse effects were common with both doses.
- Semaglutide (Ozempic) – another injectable GLP-1 receptor agonist for type 2 diabetes. Med Lett Drugs Ther 2018; 60:19.
- Drugs for type 2 diabetes. Med Lett Drugs Ther 2019; 61:169.
- Oral semaglutide (Rybelsus) for type 2 diabetes. Med Lett Drugs Ther 2019; 61:166.
- Semaglutide (Wegovy) for weight loss. Med Lett Drugs Ther 2021; 63:106.
- JP Frias et al. Efficacy and safety of once-weekly semaglutide 2.0 mg versus 1.0 mg in patients with type 2 diabetes (SUSTAIN FORTE): a double-blind, randomised, phase 3B trial. Lancet Diabetes Endocrinol 2021; 9:563.