ISSUE1671
- Mark Abramowicz, M.D., President has disclosed no relevant financial relationships.
- Jean-Marie Pflomm, Pharm.D., Editor in Chief has disclosed no relevant financial relationships.
- Brinda M. Shah, Pharm.D., Consulting Editor has disclosed no relevant financial relationships.
- Michael Viscusi, Pharm.D., Associate Editor has disclosed no relevant financial relationships.
- Discuss the efficacy of available COVID-19 antivirals for outpatient treatment of COVID-19 in vaccinated patients.
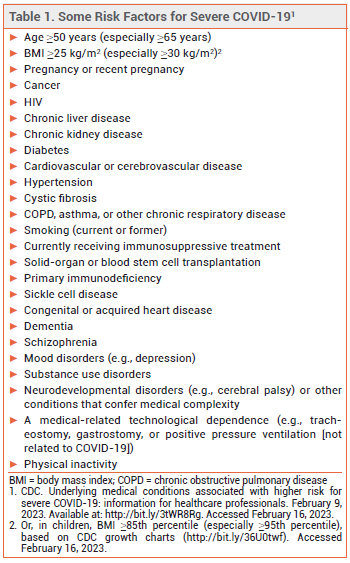
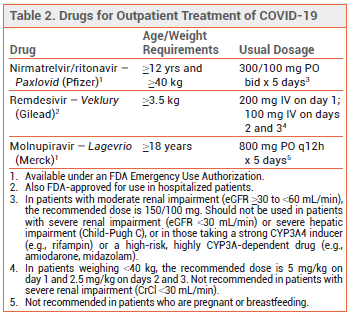
Three products are currently available in the US for treatment of high-risk (see Table 1),1 nonhospitalized adults with mild to moderate COVID-19: oral ritonavir-boosted nirmatrelvir (Paxlovid), IV remdesivir (Veklury), and oral molnupiravir (Lagevrio). Remdesivir is FDA-approved for such use; nirmatrelvir/ritonavir and molnupiravir are available under an FDA Emergency Use Authorization. Because the pivotal clinical trials of these products for outpatient use were conducted in patients who were not vaccinated against COVID-19, some clinicians have questioned whether they can benefit vaccinated outpatients.
NIRMATRELVIR/RITONAVIR — In a medical record review of about 700,000 outpatient adults with COVID-19, use of nirmatrelvir/ritonavir was associated with a ~50% reduction in the rate of hospitalization within 30 days after diagnosis in the overall population and in the subgroup of patients who had received ≥3 mRNA COVID-19 vaccine doses.2 In a database review of about 180,000 high-risk outpatients, use of nirmatrelvir/ritonavir was associated with a reduced risk of severe COVID-19 or death within 28 days in both vaccinated and unvaccinated patients.3 Similar results were observed in a database review of about 21,000 outpatient adults.4
In a cohort study of about 45,000 high-risk outpatients ≥50 years old with COVID-19, 90% of whom had received ≥3 vaccine doses, the rate of hospitalization within 14 days or death within 28 days was significantly lower in those who took nirmatrelvir/ritonavir compared to those who did not (0.52% vs 0.93%).5
In another cohort study, 1130 high-risk, vaccinated, outpatient adults with COVID-19 who received nirmatrelvir/ritonavir were compared with an equal number of matched controls. The rate of emergency department visits, hospitalization, or death within 30 days was significantly lower in the nirmatrelvir/ritonavir group (7.9% vs 14.4%).6
REMDESIVIR — Well-designed studies on use of remdesivir in vaccinated outpatients with COVID-19 are lacking. In a randomized, observer-blind trial in China, 771 high-risk outpatient adults with COVID-19 (76% vaccinated) received either an oral, deuterated remdesivir analog (VV116) or nirmatrelvir/ritonavir. Time to sustained recovery, the primary endpoint, with VV116 was noninferior to that with nirmatrelvir/ritonavir. Vaccination status did not significantly alter the relative differences between the two groups.7
MOLNUPIRAVIR — In an open-label trial in the UK, about 25,000 high-risk outpatient adults with COVID-19 were randomized to receive usual care with or without molnupiravir; about 95% of patients had previously received ≥3 COVID-19 vaccine doses. There was no significant difference between the two groups in the rate of all-cause hospitalization or death within 28 days.8
In a database review, 2661 high-risk outpatient adults with a first-time diagnosis of COVID-19 who received molnupiravir were compared with an equal number of matched controls. The rate of severe or fatal COVID-19 within 28 days, the primary endpoint, did not differ significantly between the two groups in the overall population or in the subgroup of patients who were adequately vaccinated against COVID-19, but it was significantly lower in the molnupiravir group among patients who were inadequately vaccinated.9
GUIDELINES — NIH guidelines currently recommend use of either ritonavir-boosted nirmatrelvir or remdesivir in high-risk, nonhospitalized adults with COVID-19; nirmatrelvir/ritonavir is preferred. If these drugs are inappropriate or unavailable, use of molnupiravir is recommended. According to the NIH, the efficacy of antiviral drugs for COVID-19 in vaccinated patients is unclear, but observational studies suggest a benefit. 10
CONCLUSION — Data on the effectiveness of antiviral drugs for treatment of COVID-19 in vaccinated, high-risk, outpatient adults are mixed. Ritonavir-boosted nirmatrelvir (Paxlovid) is the treatment of choice in such patients; it has been associated with improved clinical outcomes in large observational studies. Trials of remdesivir (Veklury) in vaccinated outpatients are lacking, but results from one randomized trial of an oral remdesivir analog suggest that it may be noninferior to Paxlovid. Treatment with molnupiravir (Lagevrio) was was not associated with a reduction in the rate of hospitalization or severe or fatal COVID-19 among vaccinated adults in a large randomized trial and a retrospective review.
- CDC. Underlying medical conditions associated with higher risk for severe COVID-19: information for healthcare professionals. February 9, 2023. Available at: http://bit.ly/3tWR8Rg. Accessed February 16, 2023.
- MM Shah et al. Paxlovid associated with decreased hospitalization rate among adults with COVID-19 – United States, April-September 2022. MMWR Morb Mortal Wkly Rep 2022; 71:1531. doi:10.15585/mmwr.mm7148e2
- R Najjar-Debbiny et al. Effectiveness of Paxlovid in reducing severe COVID-19 and mortality in high risk patients. Clin Infect Dis 2023; 76:e342. doi:10.1093/cid/ciac443
- NR Aggarwal et al. Real-world use of nirmatrelvir-ritonavir in outpatients with COVID-19 during the era of omicron variants including BA.4 and BA.5 in Colorado, USA: a retrospective cohort study. Lancet Infect Dis 2023 February 10 (epub). doi:10.1016/S1473-3099(23)00011-7
- S Dryden-Peterson et al. Nirmatrelvir plus ritonavir for early COVID-19 in a large U.S. health system: a population-based cohort study. Ann Intern Med 2023; 176:77. doi:10.7326/M22-2141
- S Ganatra et al. Oral nirmatrelvir and ritonavir in non-hospitalized vaccinated patients with Covid-19. Clin Infect Dis 2022 August 20 (epub). doi:10.1093/cid/ciac673
- Z Cao et al. VV116 versus nirmatrelvir–ritonavir for oral treatment of Covid-19. N Engl J Med 2023; 388:406. doi:10.1056/NEJMoa2208822
- CC Butler et al. Molnupiravir plus usual care versus usual care alone as early treatment for adults with COVID-19 at increased risk of adverse outcomes (PANORAMIC): an open-label, platform-adaptive randomised controlled trial. Lancet 2023; 401:281. doi:10.1016/S0140-6736(22)02597-1
- R Najjar-Debbiny et al. Effectiveness of molnupiravir in high risk patients: a propensity score matched analysis. Clin Infect Dis 2023; 76:453. doi:10.1093/cid/ciac781
- NIH. COVID-19 treatment guidelines. Therapeutic management of nonhospitalized adults with COVID-19. December 28, 2022. Available at: https://bit.ly/3w5TdLB. Accessed February 16, 2023.