ISSUE1723
- Mark Abramowicz, M.D., President has disclosed no relevant financial relationships.
- Jean-Marie Pflomm, Pharm.D., Editor in Chief has disclosed no relevant financial relationships.
- Michael Viscusi, Pharm.D., Associate Editor has disclosed no relevant financial relationships.
- Review the efficacy and safety of suzetrigine (Journavx) for treatment of moderate to severe acute pain.
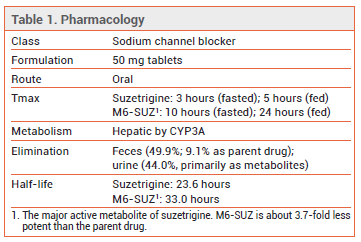
- Description: An oral sodium channel blocker.
- Indication: Treatment of moderate to severe acute pain in adults.
- Efficacy: In two 48-hour double-blind trials in adults with moderate to severe acute postoperative pain, suzetrigine was more effective than placebo. Suzetrigine was not superior to hydrocodone/acetaminophen in either trial.
- Adverse Effects: Pruritus was most common (2%).
- Drug Interactions: Suzetrigine is a CYP3A4 substrate and inducer; use with strong CYP3A4 inhibitors is contraindicated.
- Dosage: 100 mg PO x 1 dose, then 50 mg q12 hours; dosage adjustments are required for moderate hepatic impairment and when used concurrently with a moderate CYP3A4 inhibitor.
- Cost: A 7-day supply costs $232.50.
- Conclusion: Suzetrigine may be an option for treatment of moderate to severe acute pain when an NSAID or an opioid is ineffective or undesirable.
Outline
Table |
The FDA has approved suzetrigine (Journavx – Vertex), a selective sodium channel blocker, for oral treatment of moderate to severe acute pain in adults. Suzetrigine is the first sodium channel blocker to be approved in the US for this indication and the first oral nonopioid drug to be approved for treatment of pain in over 25 years.

STANDARD TREATMENT ― Nonopioid drugs are preferred for treatment of pain. In patients with moderate to severe acute pain, use of an NSAID plus acetaminophen may be at least as effective as an oral opioid combined with acetaminophen or even an injected opioid. NSAIDs can increase the risk of bleeding and can cause cardiovascular, gastrointestinal, and renal adverse effects.
As-needed use of a short-acting full opioid agonist may be required for severe acute pain. Opioids should be given in the lowest dosage and for the shortest duration possible; higher doses are associated with increased risks of motor vehicle injury, overdose, and opioid use disorder.1,2
PHARMACOLOGY ― Suzetrigine is a selective blocker of the voltage-gated sodium channel NaV1.8, which is expressed in peripheral sensory neurons such as dorsal root ganglion neurons. By stabilizing the closed state of the channel, suzetrigine inhibits the generation of action potentials that act as pain signals in the central nervous system. The drug has not been shown to cause addiction or dependence in human or animal studies.3
CLINICAL STUDIES ― FDA approval of suzetrigine was based on the results of two double-blind trials (available only as an abstract) in a total of 2191 adults with moderate to severe pain (mean score ~7 on a 0-10 scale) following full abdominoplasty (Trial 1) or bunionectomy (Trial 2). Patients were randomized to receive 48 hours of treatment with suzetrigine (100 mg initially, then 50 mg every 12 hours), hydrocodone/acetaminophen (5/325 mg every 6 hours), or placebo. Rescue treatment with ibuprofen (400 mg every 6 hours) was permitted.
The least-squares mean time-weighted sum of pain intensity differences from baseline over the first 48 hours of treatment (SPID48), the primary endpoint, was significantly greater with suzetrigine than with placebo in both Trial 1 (118.4 vs 70.1) and Trial 2 (99.9 vs 70.6). Compared to hydrocodone/acetaminophen, the mean SPID48 value with suzetrigine was similar in Trial 1 (118.4 vs 111.8) but significantly smaller in Trial 2 (99.9 vs 120.1). The median time to the onset of perceptible pain relief with suzetrigine was 30 minutes in Trial 1 and 60 minutes in Trial 2.4
ADVERSE EFFECTS ― The most common adverse effect of suzetrigine in the two 48-hour trials (occurring more often than with placebo) was pruritus (2%). Muscle spasms, rash, increased creatine phosphokinase levels, and decreased estimated glomerular filtration rate also occurred. Similar adverse effects were observed in an unpublished open-label trial in which 256 adults took suzetrigine for up to 14 days.
PREGNANCY AND LACTATION ― No data are available on the use of suzetrigine in pregnant or lactating women. In pregnant rats, exposure to suzetrigine levels 2.2 times those achieved with the maximum recommended human dose (MRHD) during organogenesis was associated with post-implantation loss and a reduced number of live fetuses, and exposure to levels 1.6 times those achieved with the MRHD during gestation and lactation was associated with increased postnatal pup mortality. Suzetrigine is secreted into animal milk.
Fertility – In a female fertility study in rats, exposure to suzetrigine levels ≥2.2 times those achieved with the MRHD was associated with a reversible increased risk of pre-implantation loss. Whether suzetrigine can reversibly affect fertility in women remains to be determined.
DRUG INTERACTIONS ― Suzetrigine and its major active metabolite are CYP3A4 substrates. Concurrent use of suzetrigine and a strong CYP3A4 inhibitor, such as ketoconazole, is contraindicated. If a moderate CYP3A4 inhibitor is coadministered, the dosage of suzetrigine should be adjusted. Products containing grapefruit can inhibit CYP3A4 and should be avoided during treatment with suzetrigine.5
Suzetrigine is also a CYP3A4 inducer; it can decrease serum concentrations and the efficacy of sensitive CYP3A4 substrates, such as midazolam. Suzetrigine did not significantly affect the pharmacokinetics of ethinyl estradiol or levonorgestrel; patients using other contraceptive hormones should use a backup method while taking suzetrigine and for 28 days after it is discontinued. Whether suzetrigine can significantly affect exposure to buprenorphine or methadone, two CYP3A4 substrates used for treatment of opioid use disorder, remains to be determined.
DOSAGE AND ADMINISTRATION ― The usual recommended dosage of suzetrigine is 100 mg once, followed by 50 mg every 12 hours thereafter for the shortest-possible duration. Taking the initial dose at least 1 hour before or 2 hours after food can accelerate the onset of analgesia; subsequent doses can be taken with or without food.
The recommended dosage of suzetrigine in patients with moderate hepatic impairment (Child-Pugh B) and in those taking a moderate CYP3A4 inhibitor concurrently is 100 mg initially, followed by 50 mg at 12, 24, and 36 hours and every 24 hours thereafter. Suzetrigine should not be used in patients with severe hepatic impairment (Child-Pugh C).
If a dose is missed, it should be taken as soon as possible and the next scheduled dose should generally be taken at the recommended time. Patients with moderate hepatic impairment and those taking a moderate CYP3A4 inhibitor concurrently should skip the next dose if it is scheduled to be taken within 6 hours.
COST ― The wholesale acquisition cost (WAC) for a 7-day supply of suzetrigine at the usual recommended dosage is $232.50.6 One pharmacoeconomic analysis has suggested that suzetrigine would be cost-saving relative to an opioid at this price because of a decreased risk of adverse effects such as opioid use disorder.7
CONCLUSION ― In two 48-hour double-blind trials in adults with moderate to severe acute postoperative pain, the oral selective sodium channel blocker suzetrigine (Journavx) was more efficacious than placebo, but not hydrocodone/acetaminophen. Suzetrigine appears to be well tolerated and to not cause addiction or dependence, but it is expensive. For most patients with moderate to severe acute pain, an NSAID should be tried first. Suzetrigine could be a reasonable alternative when NSAIDs or opioids are ineffective or the risk of adverse effects with their use is unacceptable. The efficacy and safety of suzetrigine for treatment of subacute or chronic pain is unknown.
- Nonopioid drugs for pain. Med Lett Drugs Ther 2022; 64:33.
- Opioids for pain. Med Lett Drugs Ther 2022; 64:193.
- JD Osteen et al. Pharmacology and mechanism of action of suzetrigine, a potent and selective NaV1.8 pain signal inhibitor for the treatment of moderate to severe pain. Pain Ther 2025 January 8 (epub). doi:10.1007/s40122-024-00697-0
- T Bertoch et al. Randomized, placebo-controlled, phase 3 trials of suzetrigine, a non-opioid, pain signal inhibitor for treatment of acute pain after abdominoplasty or bunionectomy. American Society of Anesthesiologists (ASA) Anesthesiology Annual Meeting 2024. Philadelphia, PA. October 18-22, 2024. Available at: https://bit.ly/3Cuq2YH. Accessed February 12, 2025.
- Inhibitors and inducers of CYP enzymes, P-glycoprotein, and other transporters. Med Lett Drugs Ther 2023 January 25 (epub). Available at: www.medicalletter.org/downloads/CYP_ PGP_Tables.pdf.
- Approximate WAC. WAC = wholesaler acquisition cost or manufacturer’s published price to wholesalers; WAC represents a published catalogue or list price and may not represent an actual transactional price. Source: AnalySource® Monthly. February 5, 2025. Reprinted with permission by First Databank, Inc. All rights reserved. ©2025. www.fdbhealth.com/policies/drug-pricing-policy.
- DM Rind et al. Suzetrigine for acute pain: effectiveness and value. Evidence report. Institute for Clinical and Economic Review, February 5, 2025. Available at: https://bit.ly/4aUbe2f. Accessed February 12, 2025.