ISSUE1725
- Mark Abramowicz, M.D., President has disclosed no relevant financial relationships.
- Jean-Marie Pflomm, Pharm.D., Editor in Chief has disclosed no relevant financial relationships.
- Amy Faucard, MLS, Associate Editor has disclosed no relevant financial relationships.
- Explain the current approach to the management of allergic rhinitis and allergic conjunctivitis.
- Discuss the pharmacologic options available for treatment of allergic rhinitis and allergic conjunctivitis and compare them based on their mechanisms of action, efficacy, and potential adverse effects.
- Determine the most appropriate therapy given the clinical presentation of a particular patient with allergic rhinitis and/or allergic conjunctivitis.
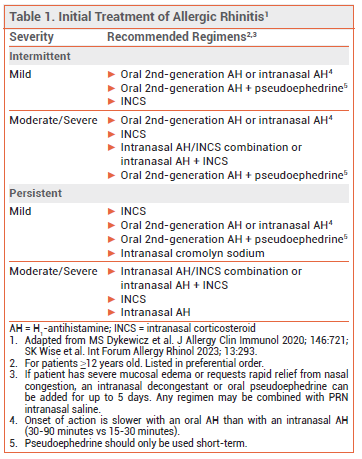
- An oral second-generation or intranasal H1-antihistamine is preferred for initial treatment of intermittent allergic rhinitis (AR).
- Intranasal corticosteroids are the most effective monotherapy for AR symptoms. They can be used in combination with an intranasal H1-antihistamine for treatment of moderate to severe symptoms.
- Use of an oral second-generation H1-antihistamine or an intranasal corticosteroid for AR also improves symptoms of allergic conjunctivitis.
- The anti-IgE monoclonal antibody omalizumab can improve symptoms in patients with poorly controlled AR (off-label use).
- Ophthalmic H1-antihistamines are at least as effective as oral second-generation H1-antihistamines for treatment of allergic conjunctivitis.
- Short-term use of an ophthalmic corticosteroid such as loteprednol can be considered for allergic conjunctivitis that fails to respond to other medications.
- Allergen immunotherapy can alter the natural history of AR, induce long-term remission, and reduce medication use.
Initial treatment of allergic rhinitis (AR) depends on the severity of symptoms and whether they are intermittent or persistent (see Table 1).1
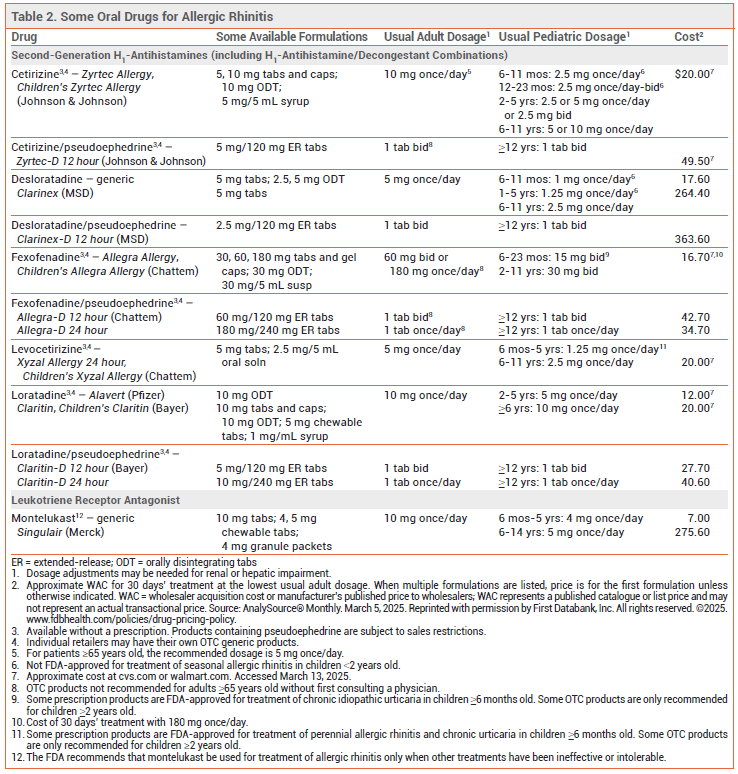
H1-ANTIHISTAMINES — Oral second-generation H1-antihistamines (see Table 2) are effective for initial treatment of the nasal symptoms of AR (itching, sneezing, rhinorrhea). They can also reduce ocular symptoms (itching, tearing, redness).
Oral first-generation H1-antihistamines such as diphenhydramine (Benadryl, and generics) are similar in efficacy to second-generation drugs, but they frequently cause anticholinergic effects and CNS impairment.
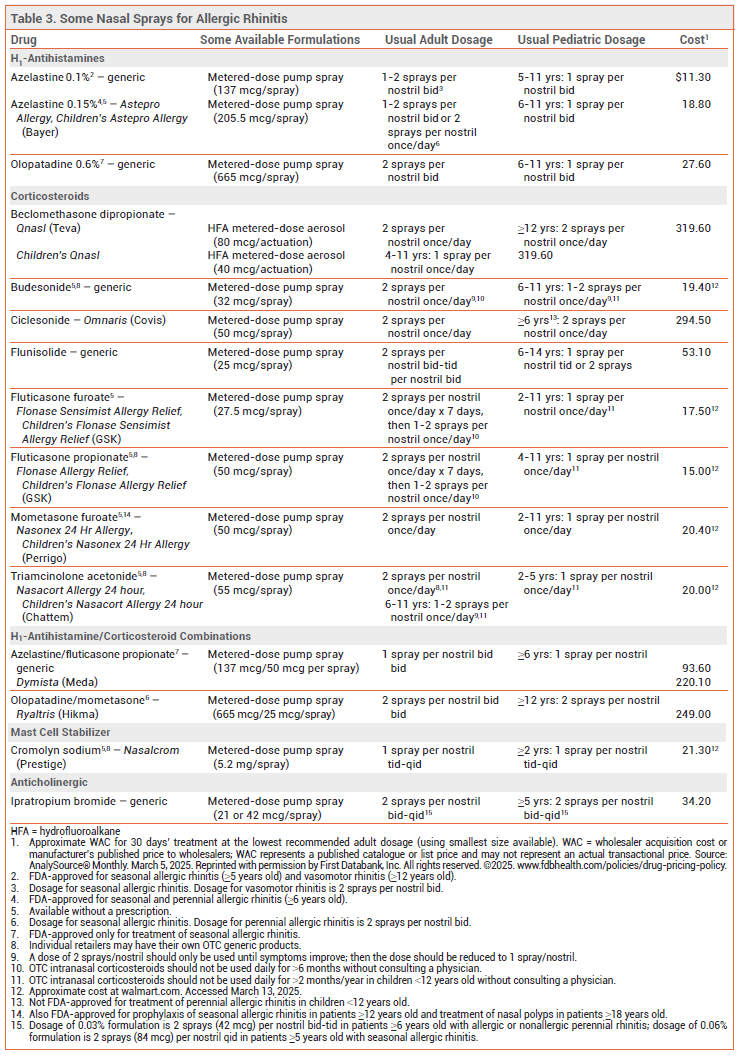
Intranasal H1-antihistamines (see Table 3) are at least as effective as oral H1-antihistamines for initial treatment of AR. Their onset of action (15-30 minutes) is more rapid than that of oral H1-antihistamines (30-90 minutes) and intranasal corticosteroids (1-12 hours). Fixed-dose combinations of an intranasal H1-antihistamine and an intranasal corticosteroid (INCS) have improved symptoms more than either drug alone in patients with seasonal AR.2,3
Adverse Effects – Oral second-generation H1-antihistamines are significantly less likely than first-generation drugs to impair CNS function and cause sedation.4 Fexofenadine (Allegra, and others) is nonsedating and does not cause CNS impairment, even in higher-than-recommended doses. Loratadine (Claritin, and others) and desloratadine (Clarinex, and generics; available only by prescription) are nonimpairing and nonsedating in recommended doses, but they may be sedating at higher doses. Cetirizine (Zyrtec, and others) and levocetirizine (Xyzal, and others) may be impairing or sedating in some patients in recommended doses.5
Oral first-generation H1-antihistamines can cause anticholinergic effects and CNS impairment with or without sedation. They can interfere with learning and memory, impair performance, and increase the risk of on-the-job injuries, motor vehicle accidents, and even plane crashes.6 When taken at night, their effects on wakefulness and psychomotor performance can persist the next day.4 The adverse effects of these drugs may be more pronounced in older adults; cumulative exposure to first-generation H1-antihistamines has been associated with an increased risk of dementia.7 In a large cohort study, use of first-generation antihistamines in children, especially those 6-24 months old, was associated with an increased risk of seizures.8
Intranasal H1-antihistamines can cause dysgeusia, dysosmia, nasal discomfort, epistaxis, and headache, and may cause somnolence.
INTRANASAL CORTICOSTEROIDS — INCSs are the most effective monotherapy for prevention and relief of the nasal symptoms of AR (itching, rhinorrhea, congestion, and sneezing). They are modestly effective in reducing ocular symptoms as well.9,10 Most INCSs are effective when used once daily. They typically begin to take effect within 2-4 hours, but maximal effects may not be achieved for 2 weeks. Patients with seasonal AR should start treatment several days before the pollen season. Several INCSs are available without a prescription (see Table 3). In patients with moderate to severe symptoms, the combination of an intranasal antihistamine and an INCS is more effective than either drug alone3; fixed-dose combinations are available.2 Adding an oral second-generation H1-antihistamine to an INCS does not increase symptom relief.1
Adverse Effects – INCSs can cause mild dryness, irritation, burning, and bleeding of the nasal mucosa, sore throat, and headache. Mucosal atrophy and septal perforation have been reported rarely; patients should be examined periodically for changes in the nasal mucosa. Intranasal and/or oropharyngeal fungal infections have occurred. One meta-analysis of randomized controlled trials in patients with AR found that INCS use was not associated with a significant risk of increased intraocular pressure or cataract formation.11 INCS use for ≥12 months in children has been associated with small decreases in growth velocity.12
MONTELUKAST — The oral leukotriene receptor antagonist montelukast (Singulair, and generics; available only by prescription) provides modest relief of nasal symptoms (itching, sneezing, rhinorrhea, and congestion). In clinical trials, the efficacy of montelukast has been equal to or less than that of an oral second-generation H1-antihistamine, and less than that of an INCS.13 Because suicidal behavior and other neuropsychiatric events continue to be reported in patients taking montelukast, it should be used for treatment of AR only when other treatments have been ineffective or intolerable.14
DECONGESTANTS — The oral decongestant pseudoephedrine and intranasal decongestants such as oxymetazoline (Afrin, and generics) act as vasoconstrictors in the nasal mucosa. They only relieve nasal congestion, not sneezing, itching, or rhinorrhea, and tolerance to their decongestant effects can develop. Oral phenylephrine (Sudafed PE, and others), which has replaced pseudoephedrine in many OTC decongestant products, has low bioavailability following oral ingestion, and the FDA has proposed removing it from oral products after a data review found that it is not effective as a nasal decongestant.15
The combination of pseudoephedrine and an oral second-generation H1-antihistamine is effective for acute relief of AR symptoms.1 Concurrent administration of an intranasal decongestant and an INCS may be considered in patients with nasal congestion that is unresponsive to an INCS alone or an intranasal H1-antihistamine/INCS combination.
Decongestants should only be used short-term. Use of intranasal decongestants for >5 consecutive days can result in rhinitis medicamentosa (rebound nasal congestion); concomitant use of an INCS may reduce this risk.1
Adverse Effects – Oral decongestants can cause insomnia, excitability, headache, nervousness, anorexia, palpitations, tachycardia, arrhythmias, hypertension, nausea, vomiting, and urinary retention. They should be used cautiously in patients with cardiovascular disease, hypertension, diabetes, migraine, hyperthyroidism, closed-angle glaucoma, or bladder neck obstruction. Use of oral decongestants is not associated with rhinitis medicamentosa.
Intranasal decongestants are less likely than oral decongestants to cause systemic adverse effects, but they can cause sneezing and stinging, burning, and dryness of the nose and throat. If rhinitis medicamentosa develops, it is usually treated by replacing the intranasal decongestant with an INCS.16
View the Comparison Table: Some Oral Drugs for Allergic Rhinitis
INTRANASAL CROMOLYN — Intranasal cromolyn (Nasalcrom) inhibits mast cell degranulation and mediator release and is mainly used before allergen exposure to prevent the onset of AR symptoms. It is particularly useful when administered just before episodic allergen exposure, such as visiting a home with cats, because it can blunt the acute allergic response. For treatment of seasonal AR, cromolyn should be started 1-2 weeks before exposure to pollen and must be taken 3-4 times daily. Intranasal cromolyn is less effective than INCSs and intranasal H1-antihistamines.1 It is relatively free from adverse effects; nasal stinging can occur.
INTRANASAL IPRATROPIUM — The antimuscarinic agent ipratropium bromide is poorly absorbed systemically and does not readily cross the blood-brain barrier. Intranasal ipratropium (available only by prescription) is effective for treatment of rhinorrhea associated with AR. It does not relieve sneezing, nasal itching, or nasal congestion. Addition of ipratropium may be beneficial in patients with rhinorrhea that is inadequately controlled with an INCS.
Adverse Effects – Ipratropium can cause dryness of the nasal and oral mucosa, epistaxis, and pharyngeal irritation. Inadvertent instillation in the eye can increase intraocular pressure.
View the Comparison Table: Some Nasal Sprays for Seasonal Allergic Rhinitis
INTRANASAL SALINE — Saline sprays and drops can relieve nasal dryness and congestion and reduce oral antihistamine use. Nasal irrigation administered by neti pot, squeeze bottle, or bulb syringe can help expel mucus and relieve congestion. Premade saline solutions and kits are available OTC. Patients should use sterile, distilled, or previously boiled water to prepare solutions for nasal irrigation.
BIOLOGICS — Biologic agents such as omalizumab (Xolair; Omlyclo) and dupilumab (Dupixent) can improve AR symptoms, but none are currently FDA-approved for such use. Omalizumab, an anti-IgE monoclonal antibody, has been studied the most. In patients with inadequately controlled AR, subcutaneous injection of omalizumab has significantly reduced rescue medication use and improved symptoms and quality of life.17 Omalizumab is generally well tolerated, but it can rarely cause anaphylaxis.
ORAL CORTICOSTEROIDS — A short course (5-7 days) of an oral corticosteroid (e.g., prednisone 20 mg qAM) can be effective for treatment of AR or rhinitis medicamentosa in patients with significant nasal obstruction that prevents adequate penetration of intranasal drugs.1
PREGNANCY — In a systematic review and meta-analysis of 37 studies that included >50,000 pregnant women, use of H1-antihistamines during the first trimester was not associated with an increased risk of major malformations, spontaneous abortions, prematurity, or low birth weight.18 The oral second-generation H1-antihistamines loratadine and cetirizine have the most data supporting their safety; the results of large cohort studies suggest that fexofenadine and desloratadine are also safe for use in pregnant women.19,20
Intranasal saline, intranasal cromolyn, and INCSs are generally considered safe for use in pregnant women. Among INCSs, budesonide is preferred because it is the best studied.21 In one prospective cohort study, maternal exposure to intranasal triamcinolone during the first trimester of pregnancy was associated with an increased risk of congenital respiratory defects.22
Oral decongestants are generally not recommended for treatment of AR in pregnant women. First-trimester use has been associated with an increased risk of birth defects; one meta-analysis of 10 studies found that pseudoephedrine increased the risk of gastroschisis.23 Intranasal decongestants have been associated with pyloric stenosis and tracheoesophageal fistula when used during the first trimester and with renal collecting system anomalies when used during the second trimester.24
Allergic conjunctivitis occurs in most patients with AR. Ocular symptoms such as itching, redness, tearing, and photophobia are frequently seasonal. Nonpharmacologic management includes allergen identification and avoidance, use of refrigerated artificial tears, eyelid cleansers and cold compresses, and refraining from eye rubbing. Wearing sunglasses as a barrier to allergens can also be helpful. Management of AR with an oral second-generation H1-antihistamine or an INCS can benefit concomitant allergic conjunctivitis as well, but oral antihistamines (even second-generation agents) may reduce tear volume and induce or worsen dry eye syndrome.25
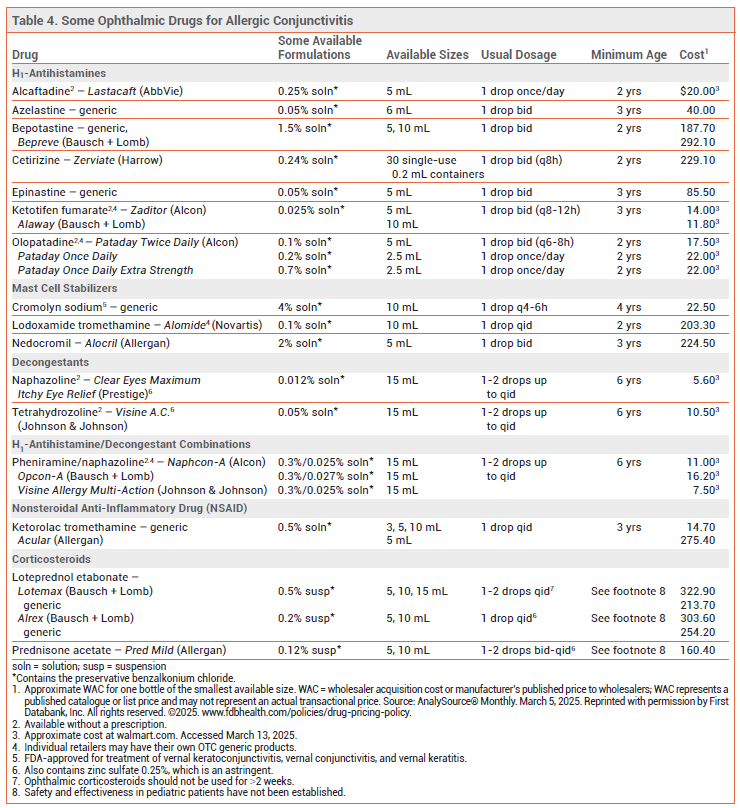
OPHTHALMIC DRUGS — Ophthalmic H1-antihistamines are at least as effective as oral second-generation H1-antihistamines for treatment of ocular itching due to allergic conjunctivitis, and they take effect within a few minutes. They are generally not effective in relieving erythema. Alcaftadine (Lastacaft), azelastine, bepotastine (Bepreve), epinastine, ketotifen (Zaditor, Alaway), and olopatadine (Pataday) also have mast cell-stabilizing effects. Starting treatment before the pollen season may improve symptom control. Alcaftadine,26 ketotifen, and olopatadine are available without a prescription (see Table 4).
The ophthalmic mast cell stabilizers cromolyn, lodoxamide (Alomide), and nedocromil (Alocril) can alleviate ocular symptoms, but they have a slower onset of action than ophthalmic H1-antihistamines and are mostly used for prevention of symptoms.
The ophthalmic nonsteroidal anti-inflammatory drug (NSAID) ketorolac (Acular, and generics) is less effective in relieving ocular symptoms than an ophthalmic H1-antihistamine.
Ophthalmic vasoconstrictors such as naphazoline and tetrahydrozoline, which are available OTC, reduce erythema, congestion, itching, and eyelid edema, but they have a short duration of action and can cause burning and stinging. They should only be used short-term (<2 weeks) or intermittently to avoid rebound hyperemia. Pheniramine/naphazoline, an OTC ophthalmic H1-antihistamine/vasoconstrictor combination, has similar adverse effects.
Ophthalmic corticosteroids can be considered for short-term use (1-2 weeks) in allergic conjunctivitis that does not respond to other drugs. Loteprednol (Lotemax, and others) is rapidly inactivated in the anterior chamber of the eye and has been associated with significantly lower rates of intraocular pressure elevation than ophthalmic prednisolone or dexamethasone.27 Dextenza, an ophthalmic insert that releases dexamethasone for up to 30 days, has been approved by the FDA for treatment of ocular itching associated with allergic conjunctivitis.28 Patients treated with ophthalmic corticosteroids should be monitored for exacerbations of conjunctival or corneal viral infections and for increased intraocular pressure. With longer treatment, cataract formation is a concern.
Adverse Effects – Most ophthalmic products available for treatment of allergic conjunctivitis contain the preservative benzalkonium chloride, which can cause burning and stinging, and may be absorbed by contact lenses. Refrigerating topical ophthalmic preparations before use is helpful. Compounding pharmacies can prepare preservative-free formulations of ophthalmic drugs.
Immunotherapy can alter the natural history of allergic respiratory disease, including rhinitis, and induce long-term remission. It should be considered for patients with moderate to severe AR who have specific IgE antibodies to clinically relevant allergens.
Subcutaneous immunotherapy (SCIT) can be formulated to contain all relevant allergens for an individual patient. Sublingual immunotherapy (SLIT) tablets are FDA-approved for treatment of AR, with or without conjunctivitis, induced by grass pollen, ragweed pollen, and dust mites.29 Aqueous formulations of SLIT have been used off-label; adequate data on their efficacy and safety are lacking.1 Both SCIT and SLIT can, in appropriately selected patients, reduce symptoms and medication use in patients with AR or allergic rhinoconjunctivitis. A systematic review of controlled trials found that SCIT and SLIT appear to be similarly effective in adults with AR, but more direct comparisons are needed.30 In indirect comparisons of randomized controlled trials, SCIT and SLIT appeared to be at least as effective as an INCS or oral antihistamine for treatment of AR.1,31
Intralymphatic immunotherapy (ILIT), an investigational route of allergen immunotherapy with a shorter treatment duration, has shown short-term benefits for treatment of seasonal allergic rhinoconjunctivitis; its long-term effects are unknown.32
Adverse Effects – Local adverse effects of SCIT include pain, redness, and swelling at the injection sites. Anaphylaxis and, very rarely, death can occur, especially in patients with uncontrolled asthma.33 SCIT should only be administered under medical supervision. After gradual dose buildup over a few months, maintenance injections are typically continued at 2- to 4-week intervals for 3-5 years.
SLIT is less likely to cause severe systemic reactions than SCIT and maintenance treatment can be self-administered at home. It can cause local adverse effects such as mouth and ear pruritus, mouth edema, and throat irritation, including eosinophilic esophagitis. Systemic adverse effects include nausea and mild abdominal pain. Anaphylaxis is rare and fatalities have not been reported.33
Pregnancy – Immunotherapy should generally not be started during pregnancy, but it can be continued at a stable dose. A large cohort study found no evidence of congenital malformations or other adverse pregnancy outcomes in women treated with SCIT or SLIT during pregnancy. 34
Additional Content Available Online
Comparison Table: Some Oral Drugs for Allergic Rhinitis
Comparison Table: Some Nasal Sprays for Seasonal Allergic Rhinitis
- SK Wise et al. International consensus statement on allergy and rhinology: allergic rhinitis – 2023. Int Forum Allergy Rhinol 2023; 13:293. doi:10.1002/alr.23090
- Olopatadine/mometasone (Ryaltris) for allergic rhinitis. Med Lett Drugs Ther 2023; 65:12.
- W Chitsuthipakorn et al. Combined medical therapy in the treatment of allergic rhinitis: systematic review and meta-analysis. Int Forum Allergy Rhinol 2022; 12:1480. doi:10.1002/alr.23015
- FER Simons and KJ Simons. Histamine and H1-antihistamines: celebrating a century of progress. J Allergy Clin Immunol 2011; 128:1139. doi:10.1016/j.jaci.2011.09.005
- H Kawauchi et al. Antihistamines for allergic rhinitis treatment from the viewpoint of nonsedative properties. Int J Mol Sci 2019; 20:213. doi:10.3390/ijms20010213
- MP McKay and L Groff. 23 years of toxicology testing fatally injured pilots: implications for aviation and other modes of transportation. Accid Anal Prev 2016; 90:108. doi:10.1016/j.aap.2016.02.008
- M Taylor-Rowan et al. Anticholinergic burden (prognostic factor) for prediction of dementia or cognitive decline in older adults with no known cognitive syndrome. Cochrane Database Syst Rev 2021; 5:CD013540. doi:10.1002/14651858.cd013540. pub2
- JH Kim et al. First-generation antihistamines and seizures in young children. JAMA Netw Open 2024; 7:e2429654. doi:10.1001/jamanetworkopen.2024.29654
- P Ratner et al. Efficacy of daily intranasal fluticasone propionate on ocular symptoms associated with seasonal allergic rhinitis. Ann Allergy Asthma Immunol 2015; 114:141. doi:10.1016/j.anai.2014.11.012
- OTC fluticasone furoate nasal spray (Flonase Sensimist) for allergic rhinitis. Med Lett Drugs Ther 2017; 59:e70.
- CV Valenzuela et al. Intranasal corticosteroids do not lead to ocular changes: a systematic review and meta-analysis. Laryngoscope 2019; 129:6. doi:10.1002/lary.27209
- DJ Mener et al. Topical intranasal corticosteroids and growth velocity in children: a meta-analysis. Int Forum Allergy Rhinol 2015; 5:95. doi:10.1002/alr.21430
- M Krishnamoorthy et al. Efficacy of montelukast in allergic rhinitis treatment: a systematic review and meta-analysis. Drugs 2020; 80:1831. doi:10.1007/s40265-020-01406-9
- In brief: Neuropsychiatric events with montelukast. Med Lett Drugs Ther 2020; 62:65.
- FDA Briefing Document. Efficacy of oral phenylephrine as a nasal decongestant. Nonprescription Drugs Advisory Committee meeting, September 11 and 12, 2023. Available at: https://bit.ly/4in4MmN. Accessed March 13, 2025.
- J Fowler et al. Rhinitis medicamentosa: a nationwide survey of Canadian otolaryngologists. J Otolaryngol Head Neck Surg 2019; 48:70. doi:10.1186/s40463-019-0392-1
- C Yu et al. Clinical efficacy and safety of omalizumab in the treatment of allergic rhinitis: a systematic review and meta-analysis of randomized clinical trials. Am J Rhinol Allergy 2020; 34:196. doi:10.1177/1945892419884774
- F Etwel et al. The risk of adverse pregnancy outcome after first trimester exposure to H1 antihistamines: a systematic review and meta-analysis. Drug Saf 2017; 40:121. doi:10.1007/s40264-016-0479-9
- NW Andersson et al. Association between fexofenadine use during pregnancy and fetal outcomes. JAMA Pediatr 2020; 174:e201316. doi:10.1001/jamapediatrics.2020.1316
- NW Andersson et al. Desloratadine use during pregnancy and risk of adverse fetal outcomes: a nationwide cohort study. J Allergy Clin Immunol Pract 2020; 8:1598. doi:10.1016/j.jaip.2020.02.017
- I Pali-Schöll et al. Allergic diseases and asthma in pregnancy, a secondary publication. World Allergy Organ J 2017; 10:10. doi:10.1186/s40413-017-0141-8
- A Berard et al. Intranasal triamcinolone use during pregnancy and the risk of adverse pregnancy outcomes. J Allergy Clin Immunol 2016; 138:97. doi:10.1016/j.jaci.2016.01.021
- S Baldacci et al. Medication use during pregnancy and the risk of gastroschisis: a systematic review and meta-analysis of observational studies. Orphanet J Rare Dis 2024; 19:31. doi:10.1186/s13023-023-02992-z
- W-P Yau et al. Use of decongestants during pregnancy and the risk of birth defects. Am J Epidemiol 2013; 178:198. doi:10.1093/aje/kws427
- AY Cheung et al. Conjunctivitis preferred practice pattern. Ophthalmology 2024; 131:P134. doi:10.1016/j.ophtha.2023.12.037
- In brief: OTC alcaftadine (Lastacaft Once Daily Relief) for allergic conjunctivitis. Med Lett Drugs Ther 2022; 64:78.
- JD Sheppard et al. Impact of the topical ophthalmic corticosteroid loteprednol etabonate on intraocular pressure. Adv Ther 2016; 33:532. doi:10.1007/s12325-016-0315-8
- A dexamethasone ophthalmic insert (Dextenza) for allergic conjunctivitis. Med Lett Drugs Ther 2023; 65:45.
- Odactra ― sublingual immunotherapy for house dust mite-induced allergic rhinitis. Med Lett Drugs Ther 2018; 60:37.
- K Tie et al. Subcutaneous versus sublingual immunotherapy for adults with allergic rhinitis: a systematic review with meta-analyses. Laryngoscope 2022; 132:499. doi:10.1002/lary.29586
- SR Durham et al. Treatment effect of sublingual immunotherapy tablets and pharmacotherapies for seasonal and perennial allergic rhinitis: pooled analyses. J Allergy Clin Immunol 2016; 138:1081. doi:10.1016/j.jaci.2016.04.061
- MP Hoang et al. Intralymphatic immunotherapy for allergic rhinoconjunctivitis: a systematic review and meta-analysis. Rhinology 2021; 59:236. doi:10.4193/rhin20.572
- DI Bernstein and TEG Epstein. Safety of allergen immunotherapy in North America from 2008-2017: lessons learned from the ACAAI/AAAAI National Surveillance Study of adverse reactions to allergen immunotherapy. Allergy Asthma Proc 2020; 41:108. doi:10.2500/aap.2020.41.200001
- N Mitselou et al. Exposure to allergen-specific immunotherapy in pregnancy and risk of congenital malformations and other adverse pregnancy outcomes. J Allergy Clin Immunol Pract 2022; 10:1635. doi:10.1016/j.jaip.2022.03.005