Matching articles for "2010"
Bromocriptine (Cycloset) for Type 2 Diabetes
The Medical Letter on Drugs and Therapeutics • December 13, 2010; (Issue 1353)
The FDA has approved a new tablet formulation of
bromocriptine mesylate (Cycloset – VeroScience) for
treatment of type 2 diabetes in adults. Bromocriptine
(Parlodel, and others) is an ergot-derived...
The FDA has approved a new tablet formulation of
bromocriptine mesylate (Cycloset – VeroScience) for
treatment of type 2 diabetes in adults. Bromocriptine
(Parlodel, and others) is an ergot-derived dopamine
agonist that has been used for more than 20 years to
treat hyperprolactinemia, acromegaly, Parkinson’s disease
and restless leg syndrome.
Oral Fingolimod (Gilenya) for Multiple Sclerosis
The Medical Letter on Drugs and Therapeutics • December 13, 2010; (Issue 1353)
The FDA has approved the marketing of fingolimod
(Gilenya – Novartis) to reduce the frequency of clinical
exacerbations and delay the accumulation of physical
disability in patients with relapsing forms of...
The FDA has approved the marketing of fingolimod
(Gilenya – Novartis) to reduce the frequency of clinical
exacerbations and delay the accumulation of physical
disability in patients with relapsing forms of multiple
sclerosis (MS). Fingolimod is the first oral drug
approved for this indication.
Drugs for Female Sexual Dysfunction
The Medical Letter on Drugs and Therapeutics • December 13, 2010; (Issue 1353)
Sexual complaints related to desire, arousal, orgasm
and painful intercourse are common in women. Since
the last Medical Letter article on this subject, some
new information has become...
Sexual complaints related to desire, arousal, orgasm
and painful intercourse are common in women. Since
the last Medical Letter article on this subject, some
new information has become available.
Clindamycin-Tretinoin (Veltin Gel) for Acne
The Medical Letter on Drugs and Therapeutics • December 13, 2010; (Issue 1353)
Veltin Gel (Stiefel), a fixed-dose combination of the
antibiotic clindamycin phosphate 1.2% and the retinoid
tretinoin 0.025%, has been approved by the FDA for topical
treatment of acne vulgaris in patients...
Veltin Gel (Stiefel), a fixed-dose combination of the
antibiotic clindamycin phosphate 1.2% and the retinoid
tretinoin 0.025%, has been approved by the FDA for topical
treatment of acne vulgaris in patients ≥12 years old.
Another product containing the same active ingredients
(Ziana) has been on the market since 2006.
In Brief: An Oral Contraceptive with Folate
The Medical Letter on Drugs and Therapeutics • December 13, 2010; (Issue 1353)
Six years after an FDA advisory committee recommended development of a combination tablet containing an oral contraceptive and folic acid,1 the FDA has approved Beyaz (Bayer), a combination of the oral...
Six years after an FDA advisory committee recommended development of a combination tablet containing an oral contraceptive and folic acid,1 the FDA has approved Beyaz (Bayer), a combination of the oral contraceptive Yaz2 with 451 mcg of levomefolate calcium, the primary metabolite of folic acid.3 According to the FDA, an unpublished double-blind, randomized U.S. trial in 379 healthy women 18-40 years old found that the combination increased serum folate levels. In an unpublished German study using a similar oral contraceptive/ levomefolate combination (summarized in the package insert), folate levels remained elevated for several weeks after levomefolate was stopped.4
The standard US diet provides 50-500 mcg of absorbable folate per day, but the bioavailability of folate in mixed diets varies. Folic acid in supplements is more bioavailable than folate in food.5 Supplementing the diet of women of childbearing age with 400 mcg of folic acid per day, the amount contained in most multivitamin preparations, has dramatically decreased the incidence of neural tube defects in their offspring.To effectively prevent neural tube defects, folic acid supplementation should be started at least one month before conception and continued through the first 2-3 months of pregnancy. Since incorrect or inconsistent use of oral contraceptives may account for as many as one million pregnancies in the US each year, all women capable of becoming pregnant should take a folic acid supplement.6,7
1. Folic acid supplementation to prevent neural tube defects. Med Lett Drugs Ther 2004; 46:17.
2. Three new oral contraceptives. Med Lett Drugs Ther 2006; 48:77.
3. Choice of contraceptives. Treat Guidel Med Lett 2010; 8:89.
4. FDA approves combination contraceptive containing a folate. http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm227237.htm. Accessed October 5, 2010.
5. GP Oakley, Jr. Eat right and take a multivitamin. N Engl J Med 1998; 338:1060.
6. Folic acid for the prevention of neural tube defects: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med 2009; 150:626.
7. AM Kaunitz. Oral contraceptive use, pregnancy intendedness and folic acid intake. FDA Advisory Committee for Reproductive Health Drugs meeting; December 15, 2003; Gaithersburg, MD. Available at http://www.fda.gov/ohrms/dockets/ac/03/transcripts/4002T1.DOC. Accessed November 3, 2010.
Download U.S. English
The standard US diet provides 50-500 mcg of absorbable folate per day, but the bioavailability of folate in mixed diets varies. Folic acid in supplements is more bioavailable than folate in food.5 Supplementing the diet of women of childbearing age with 400 mcg of folic acid per day, the amount contained in most multivitamin preparations, has dramatically decreased the incidence of neural tube defects in their offspring.To effectively prevent neural tube defects, folic acid supplementation should be started at least one month before conception and continued through the first 2-3 months of pregnancy. Since incorrect or inconsistent use of oral contraceptives may account for as many as one million pregnancies in the US each year, all women capable of becoming pregnant should take a folic acid supplement.6,7
1. Folic acid supplementation to prevent neural tube defects. Med Lett Drugs Ther 2004; 46:17.
2. Three new oral contraceptives. Med Lett Drugs Ther 2006; 48:77.
3. Choice of contraceptives. Treat Guidel Med Lett 2010; 8:89.
4. FDA approves combination contraceptive containing a folate. http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm227237.htm. Accessed October 5, 2010.
5. GP Oakley, Jr. Eat right and take a multivitamin. N Engl J Med 1998; 338:1060.
6. Folic acid for the prevention of neural tube defects: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med 2009; 150:626.
7. AM Kaunitz. Oral contraceptive use, pregnancy intendedness and folic acid intake. FDA Advisory Committee for Reproductive Health Drugs meeting; December 15, 2003; Gaithersburg, MD. Available at http://www.fda.gov/ohrms/dockets/ac/03/transcripts/4002T1.DOC. Accessed November 3, 2010.
Download U.S. English
In Brief: Clopidogrel and Omeprazole
The Medical Letter on Drugs and Therapeutics • November 29, 2010; (Issue 1352)
Use of a proton pump inhibitor (PPI) to protect against gastrointestinal (GI) bleeding in patients taking the antiplatelet agent clopidogrel (Plavix) may interfere with the activation of clopidogrel and...
Use of a proton pump inhibitor (PPI) to protect against gastrointestinal (GI) bleeding in patients taking the antiplatelet agent clopidogrel (Plavix) may interfere with the activation of clopidogrel and diminish its antiplatelet effect, increasing the risk of cardiovascular events.1 A randomized, placebo-controlled trial (COGENT) has found that use of the PPI omeprazole in patients taking clopidogrel in addition to aspirin decreased the incidence of GI bleeding without increasing the risk of a cardiovascular event, but the number of cardiovascular events was small and the formulation of omeprazole was atypical.2 The FDA in the same issue of the same journal cautioned against concluding from the results of COGENT that concurrent use of clopidogrel and omeprazole is safe.3
To some extent, all PPIs reduce the enzymatic activity of CYP2C19, which is thought to be mainly responsible for the bioactivation of clopidogrel. Omeprazole is a strong inhibitor of CYP2C19; pantoprazole (Protonix, and others) appears to have less effect on CYP2C19 and not to attenuate the antiplatelet effect of clopidogrel.4-6 Medical Letter consultants believe that patients at risk for upper GI bleeding who take clopidogrel should also take a PPI, but not omeprazole. Until more data become available on other PPIs, pantoprazole would be a reasonable choice.
1. PPI interactions with clopidogrel revisited. Med Lett Drugs Ther 2009; 51:13.
2. DL Bhatt et al. Clopidogrel with or without omeprazole in coronary artery disease. N Engl J Med 2010; 363:1909.
3. MR Southworth and R Temple. Interaction of clopidogrel and omeprazole. N Engl J Med 2010; 363:1977.
4. DJ Angiolillo et al. Differential effects of omeprazole and pantoprazole on the pharmacodynamics and pharmacokinetics of clopidogrel in healthy subjects: randomized, placebo-controlled, crossover comparison studies. Clin Pharmacol Ther 2010; Sept 15 epub.
5. T Cuisset et al. Comparison of omeprazole and pantoprazole influence on a high 150-mg clopidogrel maintenance dose: the PACA (Proton Pump Inhibitors And Clopidogrel Association) prospective randomized study. J Am Coll Cardiol 2009; 54:1149.
6. H Neubaurer et al. Pantoprazole does not influence the antiplatelet effect of clopidogrel – a whole blood aggregometry study after coronary stenting. J Cardiovasc Pharmacol 2010; 56:91.
Download U.S. English
To some extent, all PPIs reduce the enzymatic activity of CYP2C19, which is thought to be mainly responsible for the bioactivation of clopidogrel. Omeprazole is a strong inhibitor of CYP2C19; pantoprazole (Protonix, and others) appears to have less effect on CYP2C19 and not to attenuate the antiplatelet effect of clopidogrel.4-6 Medical Letter consultants believe that patients at risk for upper GI bleeding who take clopidogrel should also take a PPI, but not omeprazole. Until more data become available on other PPIs, pantoprazole would be a reasonable choice.
1. PPI interactions with clopidogrel revisited. Med Lett Drugs Ther 2009; 51:13.
2. DL Bhatt et al. Clopidogrel with or without omeprazole in coronary artery disease. N Engl J Med 2010; 363:1909.
3. MR Southworth and R Temple. Interaction of clopidogrel and omeprazole. N Engl J Med 2010; 363:1977.
4. DJ Angiolillo et al. Differential effects of omeprazole and pantoprazole on the pharmacodynamics and pharmacokinetics of clopidogrel in healthy subjects: randomized, placebo-controlled, crossover comparison studies. Clin Pharmacol Ther 2010; Sept 15 epub.
5. T Cuisset et al. Comparison of omeprazole and pantoprazole influence on a high 150-mg clopidogrel maintenance dose: the PACA (Proton Pump Inhibitors And Clopidogrel Association) prospective randomized study. J Am Coll Cardiol 2009; 54:1149.
6. H Neubaurer et al. Pantoprazole does not influence the antiplatelet effect of clopidogrel – a whole blood aggregometry study after coronary stenting. J Cardiovasc Pharmacol 2010; 56:91.
Download U.S. English
Drugs for Kidney Stones
The Medical Letter on Drugs and Therapeutics • November 29, 2010; (Issue 1352)
Renal colic is a common problem in emergency departments. Stones <5 mm in diameter often pass spontaneously; stones >10 mm in diameter generally do not. The usual treatment for stones that do not pass is...
Renal colic is a common problem in emergency departments. Stones <5 mm in diameter often pass spontaneously; stones >10 mm in diameter generally do not. The usual treatment for stones that do not pass is ureteroscopy with laser lithotripsy or shockwave lithotripsy. Some clinicians have suggested that off-label use of an oral alpha-adrenergic blocker such as tamsulosin (Flomax, and others) or calcium channel blocker such as nifedipine (Procardia XL, and others) could be tried first, with or without a corticosteroid. Both adrenoreceptors and calcium channels may have a role in the contractility of the ureter.
Aliskiren/Amlodipine (Tekamlo): Another Combination Tablet for Hypertension
The Medical Letter on Drugs and Therapeutics • November 29, 2010; (Issue 1352)
The FDA has approved Tekamlo (Novartis), an oral
fixed-dose combination of the direct renin inhibitor
aliskiren (Tekturna) and the calcium channel blocker
amlodipine (Norvasc, and others), for treatment...
The FDA has approved Tekamlo (Novartis), an oral
fixed-dose combination of the direct renin inhibitor
aliskiren (Tekturna) and the calcium channel blocker
amlodipine (Norvasc, and others), for treatment of
hypertension in patients not adequately controlled on
monotherapy or already taking both drugs, and as initial
therapy in those likely to need multiple drugs to control
their blood pressure (BP). Both aliskiren and amlodipine
are available in combinations with other antihypertensive
agents.
Miconazole (Oravig) for Oropharyngeal Candidiasis
The Medical Letter on Drugs and Therapeutics • November 29, 2010; (Issue 1352)
The FDA has approved a buccal tablet formulation of
miconazole (Oravig – Strativa) for local treatment of
oropharyngeal candidiasis in adults. Miconazole has
been available for many years in topical...
The FDA has approved a buccal tablet formulation of
miconazole (Oravig – Strativa) for local treatment of
oropharyngeal candidiasis in adults. Miconazole has
been available for many years in topical formulations
for treatment of superficial fungal infections and vulvovaginal
candidiasis.
A New Botulinum Toxin (Xeomin) for Cervical Dystonia and Blepharospasm
The Medical Letter on Drugs and Therapeutics • November 15, 2010; (Issue 1351)
The FDA has approved incobotulinumtoxinA (Xeomin –
Merz) for treatment of cervical dystonia and blepharospasm
in adults. It has been commercially available
in Germany since 2005. Several formulations...
The FDA has approved incobotulinumtoxinA (Xeomin –
Merz) for treatment of cervical dystonia and blepharospasm
in adults. It has been commercially available
in Germany since 2005. Several formulations of
botulinum toxin type A (Botox; Dysport) and type B
(Myobloc) are already marketed for treatment of cervical
dystonia. Botox is also approved for treatment of blepharospasm.
Desirudin (Iprivask) for DVT Prevention
The Medical Letter on Drugs and Therapeutics • November 1, 2010; (Issue 1350)
The injectable direct thrombin inhibitor desirudin (Iprivask – Canyon), a recombinant analog of hirudin,
the leech anticoagulant protein, was approved by the FDA in 2003 for prevention of venous...
The injectable direct thrombin inhibitor desirudin (Iprivask – Canyon), a recombinant analog of hirudin,
the leech anticoagulant protein, was approved by the FDA in 2003 for prevention of venous thromboembolism (VTE) after elective hip arthroplasty, but was only marketed recently in the US. It has been available in Europe as Revasc for about 10 years. Two other hirudin analogs are available in the US: lepirudin (Refludan) for treatment of heparin-induced thrombocytopenia (HIT) and bivalirudin (Angiomax) for use in percutaneous coronary intervention (PCI).
Interferon Beta-1b (Extavia) for Multiple Sclerosis
The Medical Letter on Drugs and Therapeutics • November 1, 2010; (Issue 1350)
The FDA has approved a new interferon beta-1b product (Extavia – Novartis) for treatment of relapsing
forms of multiple sclerosis (MS). Extavia is identical to Betaseron (Bayer); both are produced in the...
The FDA has approved a new interferon beta-1b product (Extavia – Novartis) for treatment of relapsing
forms of multiple sclerosis (MS). Extavia is identical to Betaseron (Bayer); both are produced in the same factory and packaged separately.
Rifaximin (Xifaxan 550) for Hepatic Encephalopathy
The Medical Letter on Drugs and Therapeutics • November 1, 2010; (Issue 1350)
The FDA has approved a new 550-mg tablet of rifaximin (Xifaxan – Salix), a minimally absorbed oral
antibiotic, to reduce the risk of recurrent hepatic encephalopathy (HE). A 200-mg tablet has been...
The FDA has approved a new 550-mg tablet of rifaximin (Xifaxan – Salix), a minimally absorbed oral
antibiotic, to reduce the risk of recurrent hepatic encephalopathy (HE). A 200-mg tablet has been available
for treatment of travelers’ diarrhea since 2004.
In Brief: Sibutramine (Meridia) Withdrawn
The Medical Letter on Drugs and Therapeutics • November 1, 2010; (Issue 1350)
The results of a postmarketing study of its cardiovascular safety have led to the removal of the weightloss drug sibutramine (Meridia) from the market in the US and Canada. It has also been withdrawn in Europe...
The results of a postmarketing study of its cardiovascular safety have led to the removal of the weightloss drug sibutramine (Meridia) from the market in the US and Canada. It has also been withdrawn in Europe and Australia, but remains on the market in many other countries. The study that led the FDA to ask Abbott Laboratories to withdraw the drug randomized 10,744 overweight patients with cardiovascular disease, diabetes or both to sibutramine or placebo for a mean duration of 3.4 years. The primary endpoint (non-fatal myocardial infarction, nonfatal stroke, cardiovascular death or resuscitation after cardiac arrest) occurred in 561 of 4906 patients (11.4%) taking sibutramine and in 490 of 4898 (10%) taking placebo (p = 0.02). Death from any cause occurred in 8.5% of the patients who took sibutramine and in 8.2% of those on placebo; this difference was not statistically significant.1
1. WP James et al. Effect of sibutramine on cardiovascular outcomes in overweight and obese subjects. N Engl J Med 2010; 363:905.
Download: U.S. English
1. WP James et al. Effect of sibutramine on cardiovascular outcomes in overweight and obese subjects. N Engl J Med 2010; 363:905.
Download: U.S. English
In Brief: Safety of Quinine
The Medical Letter on Drugs and Therapeutics • November 1, 2010; (Issue 1350)
Qualaquin, the only formulation of quinine sulfate available in the US, is approved only for treatment of uncomplicated malaria, but most prescriptions for its use are written for treatment or prevention of...
Qualaquin, the only formulation of quinine sulfate available in the US, is approved only for treatment of uncomplicated malaria, but most prescriptions for its use are written for treatment or prevention of nocturnal leg cramps. The FDA recently issued a warning about its safety.
Between April 2005 and October 2008, 38 cases of serious or life-threatening adverse effects of quinine were reported to the FDA. Twenty-one of these patients had thrombocytopenia and required hospitalization. Two deaths were reported: one from hemolysis and the other from thrombotic thrombocytopenic purpura (TTP). Some patients developed mucosal bleeding (gingival, gastrointestinal, epistaxis), hemoptysis, petechiae or ecchymosis. The median time to onset of adverse effects after starting quinine was about 13 days. Most patients with thrombocytopenia recovered when quinine was stopped.1 In addition to hematologic toxicity, quinine can cause cinchonism (tinnitus, headache, disturbed vision and nausea) and QT prolongation.
1. FDA drug safety communication: new risk management plan and patient medication guide for Qualaquin (quinine sulfate). Available at www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/ucm218202.htm. Accessed October 25, 2010.
Download: U.S. English
Between April 2005 and October 2008, 38 cases of serious or life-threatening adverse effects of quinine were reported to the FDA. Twenty-one of these patients had thrombocytopenia and required hospitalization. Two deaths were reported: one from hemolysis and the other from thrombotic thrombocytopenic purpura (TTP). Some patients developed mucosal bleeding (gingival, gastrointestinal, epistaxis), hemoptysis, petechiae or ecchymosis. The median time to onset of adverse effects after starting quinine was about 13 days. Most patients with thrombocytopenia recovered when quinine was stopped.1 In addition to hematologic toxicity, quinine can cause cinchonism (tinnitus, headache, disturbed vision and nausea) and QT prolongation.
1. FDA drug safety communication: new risk management plan and patient medication guide for Qualaquin (quinine sulfate). Available at www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/ucm218202.htm. Accessed October 25, 2010.
Download: U.S. English
Guanfacine Extended-Release (Intuniv) for ADHD
The Medical Letter on Drugs and Therapeutics • October 18, 2010; (Issue 1349)
An extended-release oral formulation of guanfacine
hydrochloride (Intuniv – Shire), a selective alpha2A-adrenergic
agonist, has been approved by the FDA for
treatment of attention-deficit/hyperactivity...
An extended-release oral formulation of guanfacine
hydrochloride (Intuniv – Shire), a selective alpha2A-adrenergic
agonist, has been approved by the FDA for
treatment of attention-deficit/hyperactivity disorder
(ADHD) in children 6-17 years old.
Mometasone/Formoterol (Dulera) for Asthma
The Medical Letter on Drugs and Therapeutics • October 18, 2010; (Issue 1349)
A combination of the corticosteroid mometasone furoate (Asmanex) and the long-acting beta2-agonist
(LABA) formoterol (Foradil) has become available in a
single metered-dose inhaler (Dulera – Schering)...
A combination of the corticosteroid mometasone furoate (Asmanex) and the long-acting beta2-agonist
(LABA) formoterol (Foradil) has become available in a
single metered-dose inhaler (Dulera – Schering) for
treatment of asthma in patients ≥12 years old. It is the
third corticosteroid/LABA combination inhaler to
become available for this indication in the US. None of
these combinations should be used for initial treatment
of asthma or for acute treatment of asthma symptoms.
Denosumab (Prolia) for Postmenopausal Osteoporosis
The Medical Letter on Drugs and Therapeutics • October 18, 2010; (Issue 1349)
The FDA has approved use of denosumab (Prolia –
Amgen) for treatment of osteoporosis in postmenopausal
women at high risk for...
The FDA has approved use of denosumab (Prolia –
Amgen) for treatment of osteoporosis in postmenopausal
women at high risk for fracture.
Seasonal Trivalent Influenza Vaccine for 2010-2011
The Medical Letter on Drugs and Therapeutics • October 4, 2010; (Issue 1348)
Annual vaccination against influenza A and B viruses is
the most effective method of preventing influenza. An
upcoming issue of The Medical Letter will review drugs
for chemoprophylaxis and treatment of...
Annual vaccination against influenza A and B viruses is
the most effective method of preventing influenza. An
upcoming issue of The Medical Letter will review drugs
for chemoprophylaxis and treatment of influenza.
Low-Dose Doxepin (Silenor) for Insomnia
The Medical Letter on Drugs and Therapeutics • October 4, 2010; (Issue 1348)
The FDA has approved a new low-dose formulation of
the tricyclic antidepressant doxepin (Silenor –
Somaxon) for treatment of insomnia associated with
sleep maintenance. The manufacturer claims that...
The FDA has approved a new low-dose formulation of
the tricyclic antidepressant doxepin (Silenor –
Somaxon) for treatment of insomnia associated with
sleep maintenance. The manufacturer claims that this
dose retains the hypnotic effect of doxepin, without
typical tricyclic adverse effects. Doxepin is available
generically in higher-strength capsules and in a liquid
formulation.
In Brief: Recommendation for Earlier Antibiotic Prophylaxis for Cesarean Delivery
The Medical Letter on Drugs and Therapeutics • October 4, 2010; (Issue 1348)
The American Congress of Obstetricians and Gynecologists (ACOG) has announced a new recommendation for antibiotic prophylaxis during cesarean delivery.1 Currently most women receive a single dose of...
The American Congress of Obstetricians and Gynecologists (ACOG) has announced a new recommendation for antibiotic prophylaxis during cesarean delivery.1 Currently most women receive a single dose of prophylactic antibiotics after the umbilical cord has been clamped to prevent antibiotics from crossing over to the newborn. The new recommendation is for women giving birth by cesarean section to routinely receive antibiotics within one hour before the start of surgery. In the case of an emergency cesarean delivery, prophylaxis should be started as soon as possible.
Recent studies have found a lower incidence of endometritis and wound infection with preoperative antibiotic administration compared to administration post-clamping.2-4 Whether widespread adoption of this practice could increase neonatal morbidity by masking the source of sepsis or by increasing the prevalence of resistant organisms remains to be determined.
The prophylactic antibiotic for cesarean section is cefazolin 1-2 g IV. For patients allergic to penicillins and cephalosporins, clindamycin with gentamicin would be a reasonable alternative.
1. The American College of Obstetricians and Gynecologists Committee on Obstetric Practice. Committee Opinion no. 465: Antimicrobial prophylaxis for cesarean delivery: timing of administration. Obstet Gynecol 2010; 116:791.
2. MM Constantine et al. Timing of perioperative antibiotics for cesarean delivery: a metaanalysis. Am J Obstet Gynecol 2008; 199:301.
3. FM Smaill and GML Gyte. Antibiotic prophylaxis versus no prophylaxis for preventing infection after cesarean section. Cochrane Database Syst Rev 2010: CD007482.
4. SA Sullivan et al. Administration of cefazolin prior to skin incision is superior to cefazolin at cord clamping in preventing post cesarean infectious morbidity: a randomized controlled trial. Am J Obstet Gynecol 2007; 196:455.
Download: U.S. English
Recent studies have found a lower incidence of endometritis and wound infection with preoperative antibiotic administration compared to administration post-clamping.2-4 Whether widespread adoption of this practice could increase neonatal morbidity by masking the source of sepsis or by increasing the prevalence of resistant organisms remains to be determined.
The prophylactic antibiotic for cesarean section is cefazolin 1-2 g IV. For patients allergic to penicillins and cephalosporins, clindamycin with gentamicin would be a reasonable alternative.
1. The American College of Obstetricians and Gynecologists Committee on Obstetric Practice. Committee Opinion no. 465: Antimicrobial prophylaxis for cesarean delivery: timing of administration. Obstet Gynecol 2010; 116:791.
2. MM Constantine et al. Timing of perioperative antibiotics for cesarean delivery: a metaanalysis. Am J Obstet Gynecol 2008; 199:301.
3. FM Smaill and GML Gyte. Antibiotic prophylaxis versus no prophylaxis for preventing infection after cesarean section. Cochrane Database Syst Rev 2010: CD007482.
4. SA Sullivan et al. Administration of cefazolin prior to skin incision is superior to cefazolin at cord clamping in preventing post cesarean infectious morbidity: a randomized controlled trial. Am J Obstet Gynecol 2007; 196:455.
Download: U.S. English
Dalfampridine (Ampyra) for MS
The Medical Letter on Drugs and Therapeutics • September 20, 2010; (Issue 1347)
The FDA has approved the use of dalfampridine (4-aminopyridine; Ampyra – Acorda), a potassium
channel blocker, to improve walking speed in patients with multiple sclerosis (MS). Walking speed is...
The FDA has approved the use of dalfampridine (4-aminopyridine; Ampyra – Acorda), a potassium
channel blocker, to improve walking speed in patients with multiple sclerosis (MS). Walking speed is considered
a reliable clinical measure of impairment in patients with MS.
Naproxen/Esomeprazole (Vimovo)
The Medical Letter on Drugs and Therapeutics • September 20, 2010; (Issue 1347)
The FDA has approved the marketing of Vimovo
(AstraZeneca), a fixed-dose combination of the nonsteroidal
anti-inflammatory drug (NSAID) naproxen
and the proton pump inhibitor (PPI) esomeprazole,...
The FDA has approved the marketing of Vimovo
(AstraZeneca), a fixed-dose combination of the nonsteroidal
anti-inflammatory drug (NSAID) naproxen
and the proton pump inhibitor (PPI) esomeprazole, for
symptomatic relief of osteoarthritis, rheumatoid arthritis
and ankylosing spondylitis and to decrease the risk
of developing gastric ulcers in patients at risk for
NSAID-associated ulcers.
Nonstandard Uses of Chelation Therapy
The Medical Letter on Drugs and Therapeutics • September 20, 2010; (Issue 1347)
Chelation therapy involves oral administration, intravenous
infusion or intramuscular injection of drugs that
increase excretion of heavy metals. The Medical Letter’s
last article on this subject found no...
Chelation therapy involves oral administration, intravenous
infusion or intramuscular injection of drugs that
increase excretion of heavy metals. The Medical Letter’s
last article on this subject found no evidence that it was
effective for treatment of cardiovascular disease. Since
then, off-label use of chelation therapy has expanded to
include treating children with autism and adults with
Alzheimer’s disease, cancer and other chronic
diseases.
Click here to view the free full article.
Click here to view the free full article.
Tribenzor for Hypertension
The Medical Letter on Drugs and Therapeutics • September 6, 2010; (Issue 1346)
Many patients with hypertension require more than one drug to control their blood pressure. Tribenzor (Daiichi Sankyo), recently approved by the FDA for treatment of hypertension, combines the calcium channel...
Many patients with hypertension require more than one drug to control their blood pressure. Tribenzor (Daiichi Sankyo), recently approved by the FDA for treatment of hypertension, combines the calcium channel blocker amlodipine (Norvasc, and others), the angiotensin receptor blocker (ARB) olmesartan (Benicar)and the most commonly prescribed diuretic, hydrochlorothiazide (HCTZ). Tribenzor is not approved for initial therapy, but is recommended for patients not adequately controlled on any 2-drug combination of a calcium channel blocker, an ARB or a diuretic.
Natazia - A New Oral Contraceptive
The Medical Letter on Drugs and Therapeutics • September 6, 2010; (Issue 1346)
The FDA has approved the marketing of Natazia (Bayer), a 4-phase oral contraceptive containing the
estrogen estradiol valerate and the progestin dienogest, both used for the first time in the US for...
The FDA has approved the marketing of Natazia (Bayer), a 4-phase oral contraceptive containing the
estrogen estradiol valerate and the progestin dienogest, both used for the first time in the US for this
indication.
In Brief: Propoxyphene Toxicity
The Medical Letter on Drugs and Therapeutics • September 6, 2010; (Issue 1346)
The FDA has required manufacturers of propoxyphene-containing products (Darvon, and others) to strengthen boxed warnings to include the potential for overdose.1 This action followed disclosure of fatal...
The FDA has required manufacturers of propoxyphene-containing products (Darvon, and others) to strengthen boxed warnings to include the potential for overdose.1 This action followed disclosure of fatal overdoses linked to propoxyphene-containing products taken alone or concurrently with other CNS depressants, including alcohol. Many of the overdoses occurred in patients with a history of emotional instability or suicide attempts. Accumulation of metabolites of propoxyphene can lead to central nervous system, cardiac and respiratory depression; convulsions and cardiotoxicity have occurred.
A Schedule IV controlled substance, propoxyphene is a weak full agonist opioid indicated for relief of mild to moderate pain.2 It is often prescribed in combination with acetaminophen (Darvocet, and others). One reasonable alternative would be codeine with acetaminophen; 32 mg of codeine has an analgesic effect similar to that of 65 mg of propoxyphene. Another would be 400 mg of ibuprofen, which may be more effective than either propoxyphene or codeine combined with acetaminophen.
1. FDA News Release. FDA takes actions on Darvon, other pain medications containing propoxyphene. Available at www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm170769.html. Accessed August 23, 2010.
2. Drugs for pain. Treat Guidel Med Lett 2010; 92:25.
Download: U.S. English
A Schedule IV controlled substance, propoxyphene is a weak full agonist opioid indicated for relief of mild to moderate pain.2 It is often prescribed in combination with acetaminophen (Darvocet, and others). One reasonable alternative would be codeine with acetaminophen; 32 mg of codeine has an analgesic effect similar to that of 65 mg of propoxyphene. Another would be 400 mg of ibuprofen, which may be more effective than either propoxyphene or codeine combined with acetaminophen.
1. FDA News Release. FDA takes actions on Darvon, other pain medications containing propoxyphene. Available at www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm170769.html. Accessed August 23, 2010.
2. Drugs for pain. Treat Guidel Med Lett 2010; 92:25.
Download: U.S. English
Bronchial Thermoplasty for Asthma
The Medical Letter on Drugs and Therapeutics • August 23, 2010; (Issue 1345)
The FDA has approved the marketing of the Alair Bronchial Thermoplasty System (Asthmatx) for treatment of severe persistent asthma in patients ≥18 years old whose asthma is not well controlled with inhaled...
The FDA has approved the marketing of the Alair Bronchial Thermoplasty System (Asthmatx) for treatment of severe persistent asthma in patients ≥18 years old whose asthma is not well controlled with inhaled corticosteroids and long-acting beta2-agonists.
Three New Drugs for Hereditary Angioedema
The Medical Letter on Drugs and Therapeutics • August 23, 2010; (Issue 1345)
In the past 2 years, the FDA has approved 3 new drugs for prevention or treatment of hereditary
angioedema (HAE) in non-pregnant adolescents and adults: the C1 esterase inhibitor Cinryze for prophylaxis and...
In the past 2 years, the FDA has approved 3 new drugs for prevention or treatment of hereditary
angioedema (HAE) in non-pregnant adolescents and adults: the C1 esterase inhibitor Cinryze for prophylaxis and the C1 esterase inhibitor Berinert and the kallikrein inhibitor ecallantide (Kalbitor) for treatment of acute attacks.
An Expanded Pneumococcal Vaccine (Prevnar 13) for Infants and Children
The Medical Letter on Drugs and Therapeutics • August 23, 2010; (Issue 1345)
The FDA has licensed a 13-valent conjugate pneumococcal vaccine (PCV13; Prevnar 13 – Pfizer) for
the prevention of invasive pneumococcal disease (IPD) in infants and children...
The FDA has licensed a 13-valent conjugate pneumococcal vaccine (PCV13; Prevnar 13 – Pfizer) for
the prevention of invasive pneumococcal disease (IPD) in infants and children <6 years old. It replaces
Prevnar (PCV7). An unconjugated 23-valent polysaccharide vaccine (PPSV23; Pneumovax 23 – Merck) is FDA-approved for use in adults.
Armodafinil (Nuvigil) for Wakefulness
The Medical Letter on Drugs and Therapeutics • August 9, 2010; (Issue 1344)
Armodafinil (Nuvigil – Cephalon), the R-enantiomer of the non-amphetamine stimulant modafinil (Provigil – Cephalon; Alertec – Shire in Canada), is being promoted directly to the public for treatment of...
Armodafinil (Nuvigil – Cephalon), the R-enantiomer of the non-amphetamine stimulant modafinil (Provigil – Cephalon; Alertec – Shire in Canada), is being promoted directly to the public for treatment of excessive daytime sleepiness associated with shift work.
Aztreonam for Inhalation Solution (Cayston) for Cystic Fibrosis
The Medical Letter on Drugs and Therapeutics • August 9, 2010; (Issue 1344)
The antibiotic aztreonam is now available as an inhalation solution (Cayston – Gilead) to improve respiratory symptoms in cystic fibrosis (CF) patients ≥7 years old colonized with Pseudomonas aeruginosa....
The antibiotic aztreonam is now available as an inhalation solution (Cayston – Gilead) to improve respiratory symptoms in cystic fibrosis (CF) patients ≥7 years old colonized with Pseudomonas aeruginosa. It is the second inhaled antibiotic to be FDA-approved for this indication in CF patients; the aminoglycoside tobramycin (Tobi) was the first. Inhaled antibiotics offer the advantage of high airway concentrations while minimizing systemic side effects.
Nutritional Support for Macular Degeneration
The Medical Letter on Drugs and Therapeutics • August 9, 2010; (Issue 1344)
An advertisement in a recent issue of Ophthalmology introduces PreserVision Eye Vitamin AREDS 2 Formula from Bausch & Lomb. No specific indication is mentioned, but the ad states that “…[the new...
An advertisement in a recent issue of Ophthalmology introduces PreserVision Eye Vitamin AREDS 2 Formula from Bausch & Lomb. No specific indication is mentioned, but the ad states that “…[the new product] builds on the original PreserVision Eye Vitamin AREDS Formula, the only formula proven to slow the progression of moderate-to-advanced AMD.”
Pitavastatin (Livalo) - The Seventh Statin
The Medical Letter on Drugs and Therapeutics • July 26, 2010; (Issue 1343)
The FDA has approved the marketing of pitavastatin (Livalo – Kowa), an HMG-CoA reductase inhibitor
(“statin”), for treatment of primary hyperlipidemia or mixed dyslipidemia. It has been available in...
The FDA has approved the marketing of pitavastatin (Livalo – Kowa), an HMG-CoA reductase inhibitor
(“statin”), for treatment of primary hyperlipidemia or mixed dyslipidemia. It has been available in Japan
since 2003. All of the statins now available in the US are listed in the table on page 58.
A New Conjugate Meningococcal Vaccine (Menveo)
The Medical Letter on Drugs and Therapeutics • July 26, 2010; (Issue 1343)
The FDA has approved Menveo (Novartis), a new quadrivalent conjugated polysaccharide vaccine, for
protection against disease caused by Neisseria meningitidis in people 11-55 years...
The FDA has approved Menveo (Novartis), a new quadrivalent conjugated polysaccharide vaccine, for
protection against disease caused by Neisseria meningitidis in people 11-55 years old.
Treatment of Lyme Disease
The Medical Letter on Drugs and Therapeutics • July 12, 2010; (Issue 1342)
Most cases of Lyme disease in the US occur between May and September in the Northeastern, Mid-Atlantic and North Central...
Most cases of Lyme disease in the US occur between May and September in the Northeastern, Mid-Atlantic and North Central states.
Tranexamic Acid (Lysteda) for Treatment of Menorrhagia
The Medical Letter on Drugs and Therapeutics • July 12, 2010; (Issue 1342)
The FDA has approved the use of tranexamic acid (Lysteda – Ferring), an oral antifibrinolytic, for treatment of menorrhagia. Tranexamic acid has been used for this purpose in Europe for decades, and is...
The FDA has approved the use of tranexamic acid (Lysteda – Ferring), an oral antifibrinolytic, for treatment of menorrhagia. Tranexamic acid has been used for this purpose in Europe for decades, and is available without a prescription in some countries. It has been available in the US since 1987 for use with coagulation factors in patients with hemophilia undergoing dental extractions.
Pralatrexate (Folotyn) for Peripheral T-Cell Lymphoma
The Medical Letter on Drugs and Therapeutics • July 12, 2010; (Issue 1342)
The FDA has approved pralatrexate (Folotyn – Allos), an intravenous (IV) antifolate, for treatment of adults with relapsed or refractory peripheral T-cell lymphoma (PTCL). It is the first drug approved by the...
The FDA has approved pralatrexate (Folotyn – Allos), an intravenous (IV) antifolate, for treatment of adults with relapsed or refractory peripheral T-cell lymphoma (PTCL). It is the first drug approved by the FDA specifically for this indication.
Preservation of Ovarian Function During Chemotherapy
The Medical Letter on Drugs and Therapeutics • June 28, 2010; (Issue 1341)
Chemotherapy can result in premature menopause and infertility in young women. Pretreatment fertility
counseling followed by appropriate action may prevent some of these undesirable...
Chemotherapy can result in premature menopause and infertility in young women. Pretreatment fertility
counseling followed by appropriate action may prevent some of these undesirable consequences.
A Sumatriptan Needle-Free Injector for Migraine
The Medical Letter on Drugs and Therapeutics • June 28, 2010; (Issue 1341)
Sumatriptan was first marketed in the US in 1993 as Imitrex for subcutaneous injection, followed by tablets for oral administration and then by a nasal spray. It is one of seven serotonin receptor agonists...
Sumatriptan was first marketed in the US in 1993 as Imitrex for subcutaneous injection, followed by tablets for oral administration and then by a nasal spray. It is one of seven serotonin receptor agonists (“triptans”) marketed in the US for treatment of migraine, but it is the only one available for subcutaneous injection. Now the FDA has approved Sumavel DosePro> (Zogenix), a needle-free device for delivering sumatriptan succinate to subcutaneous tissue, for treatment of migraine and cluster headache in adults.
Ofatumumab (Arzerra) for CLL
The Medical Letter on Drugs and Therapeutics • June 28, 2010; (Issue 1341)
The FDA has approved ofatumumab (Arzerra – GlaxoSmithKline), a human anti-CD20 monoclonal
antibody, for treatment of patients with chronic lymphocytic leukemia (CLL) refractory to fludarabine (Fludara, and...
The FDA has approved ofatumumab (Arzerra – GlaxoSmithKline), a human anti-CD20 monoclonal
antibody, for treatment of patients with chronic lymphocytic leukemia (CLL) refractory to fludarabine (Fludara, and others) and alemtuzumab (Campath). It is the second anti-CD20 antibody approved for treatment of CLL; rituximab (Rituxan), a chimeric murine/human antibody, was the first.
In Brief: Only The Name Remains The Same
The Medical Letter on Drugs and Therapeutics • June 28, 2010; (Issue 1341)
A Medical Letter subscriber was surprised to discover that a new Citracal product contained not only calcium citrate, but also calcium carbonate. Citracal Plus Bone Density Builder actually contains more...
A Medical Letter subscriber was surprised to discover that a new Citracal product contained not only calcium citrate, but also calcium carbonate. Citracal Plus Bone Density Builder actually contains more calcium carbonate per tablet than calcium citrate (240 mg vs. 60 mg). Another Citracal product, Citracal Plus Heart Health, also contains more calcium carbonate than calcium citrate. Many clinicians prefer calcium citrate because it can be taken with or without food, while calcium carbonate must be taken with food for optimal absorption. Other familiar over-the-counter (OTC) names also contain some surprises among their ingredients, as shown in the table below.
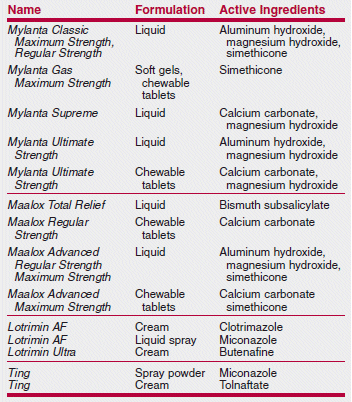
>Many well-known brand-name OTC products no longer contain only or necessarily any of their original ingredients.
Download: U.S. English
Robotic-Assisted Laparoscopic Surgery
The Medical Letter on Drugs and Therapeutics • June 14, 2010; (Issue 1340)
The da Vinci Surgical System (Intuitive Surgical, Sunnyvale, CA) has become available in recent years
for use in laparoscopic prostatectomy, gynecological procedures, general surgery, otolaryngology and...
The da Vinci Surgical System (Intuitive Surgical, Sunnyvale, CA) has become available in recent years
for use in laparoscopic prostatectomy, gynecological procedures, general surgery, otolaryngology and cardiothoracic procedures.
A Plasma-derived Von Willebrand Factor/Factor VIII Concentrate (Wilate)
The Medical Letter on Drugs and Therapeutics • June 14, 2010; (Issue 1340)
The FDA has approved a new plasma-derived von Willebrand factor/Factor VIII concentrate (Wilate –
Octapharma) for treatment of spontaneous and trauma-induced bleeding episodes in patients with...
The FDA has approved a new plasma-derived von Willebrand factor/Factor VIII concentrate (Wilate –
Octapharma) for treatment of spontaneous and trauma-induced bleeding episodes in patients with von
Willebrand disease.
Tocilizumab (Actemra) for Rheumatoid Arthritis
The Medical Letter on Drugs and Therapeutics • June 14, 2010; (Issue 1340)
The FDA has approved tocilizumab (Actemra – Genentech; RoActemra in Europe) for intravenous
(IV) treatment of adult patients with moderately to severely active rheumatoid arthritis who have had...
The FDA has approved tocilizumab (Actemra – Genentech; RoActemra in Europe) for intravenous
(IV) treatment of adult patients with moderately to severely active rheumatoid arthritis who have had an
inadequate response to tumor necrosis factor (TNF) inhibitors.
In Brief: Herpes Zoster Vaccine (Zostavax) Revisited
The Medical Letter on Drugs and Therapeutics • May 31, 2010; (Issue 1339)
The 2006 Medical Letter article on the then-new varicella-zoster vaccine concluded that Zostavax appears to be safe and effective in protecting patients ≥60 years old against herpes zoster and postherpetic...
The 2006 Medical Letter article on the then-new varicella-zoster vaccine concluded that Zostavax appears to be safe and effective in protecting patients ≥60 years old against herpes zoster and postherpetic neuralgia, especially in reducing the severity and duration of the disease.1 Some new information has recently become available.
CLINICAL STUDIES — A Veterans Administration randomized, double-blind trial enrolled more than 38,000 patients ≥60 years old and followed them for a mean of 3.4 years after administration of Zostavax or placebo. Since the efficacy of the vaccine had been demonstrated previously (51% in preventing zoster and 67% in preventing postherpetic neuralgia), the objective of this study was to examine its safety. Transient varicella-like rash occurred at the inoculation site in 0.11% of vaccine recipients and in 0.04% of patients who received a placebo injection. Erythema, swelling, pain and tenderness at the injection site were more frequent and more severe with the vaccine than with placebo. There were no other significant differences. Serious adverse events occurred in 1.4% of patients in each group.2
USE — Despite its efficacy and the frequency and morbidity of herpes zoster, this vaccine is hardly used. One study in 2007 found that only 2% of patients ≥60 years old had received it.3 A 2008 survey found that 7% of potential recipients had been vaccinated.4 A study of the reasons for such sparse usage concluded that the expense ($194 wholesale), the need for a freezer to store the vaccine (a vaccine that can be kept in a refrigerator is available in Europe), and reimbursement through Medicare Part D, which generally provides pharmacy benefits, rather than Part B, which physicians are more familiar with, were contributing factors.5
CONCLUSION — The efficacy of the herpes zoster vaccine (Zostavax) was well established before the FDA approved it in 2006. Several years’ use has now provided more data supporting the safety of the vaccine. It deserves wider use.
1. Herpes zoster vaccine (Zostavax). Med Lett Drugs Ther 2006; 48:73.
2. MS Simberkoff et al. Safety of herpes zoster vaccine in the shingles prevention study. Ann Intern Med 2010; 152:545.
3. PJ Lu et al. Herpes zoster vaccination among adults aged 60 years or older in the United States, 2007: uptake of the first new vaccine to target seniors. Vaccine 2009; 27:882.
4. JS Schiller and GL Euler. Vaccination coverage estimates from the National Health Interview Survey: United States, 2008. Atlanta: Centers for Disease Control and Prevention 2009. Accessed at www.cdc.gov/nchs/data/hestat/vaccine_coverage/vaccine_coverage.pdf on 12 May 2010.
5. LP Hurley et al. Barriers to the use of herpes zoster vaccine. Ann Intern Med 2010; 152:555.
Download: U.S. English
CLINICAL STUDIES — A Veterans Administration randomized, double-blind trial enrolled more than 38,000 patients ≥60 years old and followed them for a mean of 3.4 years after administration of Zostavax or placebo. Since the efficacy of the vaccine had been demonstrated previously (51% in preventing zoster and 67% in preventing postherpetic neuralgia), the objective of this study was to examine its safety. Transient varicella-like rash occurred at the inoculation site in 0.11% of vaccine recipients and in 0.04% of patients who received a placebo injection. Erythema, swelling, pain and tenderness at the injection site were more frequent and more severe with the vaccine than with placebo. There were no other significant differences. Serious adverse events occurred in 1.4% of patients in each group.2
USE — Despite its efficacy and the frequency and morbidity of herpes zoster, this vaccine is hardly used. One study in 2007 found that only 2% of patients ≥60 years old had received it.3 A 2008 survey found that 7% of potential recipients had been vaccinated.4 A study of the reasons for such sparse usage concluded that the expense ($194 wholesale), the need for a freezer to store the vaccine (a vaccine that can be kept in a refrigerator is available in Europe), and reimbursement through Medicare Part D, which generally provides pharmacy benefits, rather than Part B, which physicians are more familiar with, were contributing factors.5
CONCLUSION — The efficacy of the herpes zoster vaccine (Zostavax) was well established before the FDA approved it in 2006. Several years’ use has now provided more data supporting the safety of the vaccine. It deserves wider use.
1. Herpes zoster vaccine (Zostavax). Med Lett Drugs Ther 2006; 48:73.
2. MS Simberkoff et al. Safety of herpes zoster vaccine in the shingles prevention study. Ann Intern Med 2010; 152:545.
3. PJ Lu et al. Herpes zoster vaccination among adults aged 60 years or older in the United States, 2007: uptake of the first new vaccine to target seniors. Vaccine 2009; 27:882.
4. JS Schiller and GL Euler. Vaccination coverage estimates from the National Health Interview Survey: United States, 2008. Atlanta: Centers for Disease Control and Prevention 2009. Accessed at www.cdc.gov/nchs/data/hestat/vaccine_coverage/vaccine_coverage.pdf on 12 May 2010.
5. LP Hurley et al. Barriers to the use of herpes zoster vaccine. Ann Intern Med 2010; 152:555.
Download: U.S. English
Safety of Inhaled Corticosteroids in Chronic Obstructive Pulmonary Disease (COPD)
The Medical Letter on Drugs and Therapeutics • May 31, 2010; (Issue 1339)
Two combinations of an inhaled corticosteroid with an inhaled long-acting beta2-agonist are approved by the
FDA for use in patients with COPD: fluticasone/salmeterol (Advair Diskus) and...
Two combinations of an inhaled corticosteroid with an inhaled long-acting beta2-agonist are approved by the
FDA for use in patients with COPD: fluticasone/salmeterol (Advair Diskus) and budesonide/formoterol
(Symbicort). A Medical Letter reader has questioned the safety of using corticosteroid inhalers in patients
with this disorder. No single-agent inhaled corticosteroid inhaler is approved for this indication.
Romidepsin (Istodax) for Cutaneous T-Cell Lymphoma
The Medical Letter on Drugs and Therapeutics • May 31, 2010; (Issue 1339)
The FDA has approved romidepsin (Istodax – Celgene), an IV histone deacetylase (HDAC) inhibitor,
for treatment of cutaneous T-cell lymphoma (CTCL) in patients who have received at least one prior...
The FDA has approved romidepsin (Istodax – Celgene), an IV histone deacetylase (HDAC) inhibitor,
for treatment of cutaneous T-cell lymphoma (CTCL) in patients who have received at least one prior systemic
therapy. The most common types of CTCL are mycosis fungoides, a low-grade lymphoma usually confined to
the skin, and Sézary syndrome, a more aggressive disease with malignant lymphocytes in the blood. Both
can progress to fatal systemic involvement. Romidepsin is the second HDAC inhibitor approved for
this indication; vorinostat (Zolinza), an oral HDAC inhibitor, was approved earlier.
Bioidentical Hormones
The Medical Letter on Drugs and Therapeutics • May 31, 2010; (Issue 1339)
In recent years, many women have become concerned about the safety of pharmaceutical replacement
hormones for treatment of menopausal symptoms. “Bioidentical” hormone preparations, which are not approved...
In recent years, many women have become concerned about the safety of pharmaceutical replacement
hormones for treatment of menopausal symptoms. “Bioidentical” hormone preparations, which are not approved by the FDA, are heavily promoted in popular books and on TV as alternatives; these are
derivatives of soy or plant extracts, chemically modified to be structurally identical to endogenous hormones.
Most FDA-approved single-entity hormones are also derivatives of soy or plant extracts and are
structurally identical to hormones produced by the ovary.
Which TNF Inhibitor for Rheumatoid Arthritis?
The Medical Letter on Drugs and Therapeutics • May 17, 2010; (Issue 1338)
The FDA has approved five tumor necrosis factor (TNF) inhibitors for treatment of moderately to
severely active rheumatoid arthritis (RA). TNF-α is a pro-inflammatory cytokine involved in the...
The FDA has approved five tumor necrosis factor (TNF) inhibitors for treatment of moderately to
severely active rheumatoid arthritis (RA). TNF-α is a pro-inflammatory cytokine involved in the pathogenesis of many systemic inflammatory disorders.
Another Once-Daily Formulation of Tramadol (Ryzolt)
The Medical Letter on Drugs and Therapeutics • May 17, 2010; (Issue 1338)
The FDA has approved tramadol hydrochloride extended-release (Ryzolt – Purdue) for treatment of
moderate to moderately severe chronic pain in adults. Tramadol is already available in another extended-release...
The FDA has approved tramadol hydrochloride extended-release (Ryzolt – Purdue) for treatment of
moderate to moderately severe chronic pain in adults. Tramadol is already available in another extended-release formulation (Ultram ER) and in immediate-release tablets alone (Ultram, and others) and combined with acetaminophen (Ultracet, and others).
In Brief: Poor Metabolizers of Clopidogrel (Plavix)
The Medical Letter on Drugs and Therapeutics • May 3, 2010; (Issue 1337)
The FDA has required the manufacturer of Plavix, an antiplatelet drug used in addition to aspirin to prevent cardiovascular events in high-risk patients,1 to add a boxed warning to the package insert about the...
The FDA has required the manufacturer of Plavix, an antiplatelet drug used in addition to aspirin to prevent cardiovascular events in high-risk patients,1 to add a boxed warning to the package insert about the risk of a poor response to the drug in patients with genetic polymorphisms of the cytochrome P450 enzyme CYP2C19. Clopidogrel is a prodrug and CYP2C19 is mainly responsible for its bioactivation. The Medical Letter reported last year that several studies have found higher rates of cardiovascular events, including stent thrombosis, in patients with these polymorphisms taking clopidogrel.2
At least one genetic polymorphism leading to poor metabolism of clopidogrel has been reported to occur in 15% of Caucasians, 17% of African Americans and 30% of Asians.3 Since many patients take clopidogrel to protect against life-threatening events, and some continue to do so for extended periods of time, it might be worthwhile to test for these polymorphisms. Such tests, requiring small amounts of blood or saliva, are commercially available from clinical laboratories. More directly, patients who are taking clopidogrel could have platelet aggregation assays to determine whether the drug is being activated.
However, the best course of action for patients who prove to be poor metabolizers of clopidogrel is not clear. They could be treated with higher doses of clopidogrel, but the doses that would be safe and effective in such patients have not been established. Alternatively, they could be treated with prasugrel (Effient), a similar antiplatelet drug that does not require CYP2C19 for activation, instead of clopidogrel, but prasugrel has a greater effect on platelets and may cause more bleeding.4
1. Antiplatelet and anticoagulant drugs. Treat Guidel Med Lett 2008; 6:29.
2. PPI interactions with clopidogrel revisited. Med Lett Drugs Ther 2009; 51:13.
3. Z Desta et al. Clinical significance of the cytochrome P450 2C19 genetic polymorphism. Clin Pharmacokinet 2002; 41:913.
4. Prasugrel (Effient) vs. clopidogrel (Plavix). Med Lett Drugs Ther 2009; 51:69.
Download: U.S. English
At least one genetic polymorphism leading to poor metabolism of clopidogrel has been reported to occur in 15% of Caucasians, 17% of African Americans and 30% of Asians.3 Since many patients take clopidogrel to protect against life-threatening events, and some continue to do so for extended periods of time, it might be worthwhile to test for these polymorphisms. Such tests, requiring small amounts of blood or saliva, are commercially available from clinical laboratories. More directly, patients who are taking clopidogrel could have platelet aggregation assays to determine whether the drug is being activated.
However, the best course of action for patients who prove to be poor metabolizers of clopidogrel is not clear. They could be treated with higher doses of clopidogrel, but the doses that would be safe and effective in such patients have not been established. Alternatively, they could be treated with prasugrel (Effient), a similar antiplatelet drug that does not require CYP2C19 for activation, instead of clopidogrel, but prasugrel has a greater effect on platelets and may cause more bleeding.4
1. Antiplatelet and anticoagulant drugs. Treat Guidel Med Lett 2008; 6:29.
2. PPI interactions with clopidogrel revisited. Med Lett Drugs Ther 2009; 51:13.
3. Z Desta et al. Clinical significance of the cytochrome P450 2C19 genetic polymorphism. Clin Pharmacokinet 2002; 41:913.
4. Prasugrel (Effient) vs. clopidogrel (Plavix). Med Lett Drugs Ther 2009; 51:69.
Download: U.S. English
Everolimus and Pazopanib: Two New Drugs for Renal Cell Cancer
The Medical Letter on Drugs and Therapeutics • May 3, 2010; (Issue 1337)
Everolimus (Afinitor – Novartis) and pazopanib (Votrient – GlaxoSmithKline), two oral multikinase inhibitors, have been approved by the FDA for treatment of advanced renal cell carcinoma. Sunitinib (Sutent)...
Everolimus (Afinitor – Novartis) and pazopanib (Votrient – GlaxoSmithKline), two oral multikinase inhibitors, have been approved by the FDA for treatment of advanced renal cell carcinoma. Sunitinib (Sutent) and sorafenib (Nexavar), two other oral multikinase inhibitors, and temsirolimus (Torisel), an IV multikinase inhibitor, were approved earlier for the same indication.
Cost of Topical Products for Tinea Pedis
The Medical Letter on Drugs and Therapeutics • May 3, 2010; (Issue 1337)
A Medical Letter reader recently received a diagnosis of athlete’s foot and a prescription for Naftin gel, for which his pharmacy charged $145, and his insurance company required a $70 copay because this...
A Medical Letter reader recently received a diagnosis of athlete’s foot and a prescription for Naftin gel, for which his pharmacy charged $145, and his insurance company required a $70 copay because this formulation was not included in its formulary. Do patients need to pay prices like these to treat tinea pedis?
In Brief: Velaglucerase (Vpriv) for Gaucher's Disease
The Medical Letter on Drugs and Therapeutics • May 3, 2010; (Issue 1337)
The FDA has approved velaglucerase alfa (Vpriv – Shire), a new formulation of glucocerebrosidase prepared from human fibroblasts, for treatment of the nonneurologic form of Gaucher’s disease (Type 1)....
The FDA has approved velaglucerase alfa (Vpriv – Shire), a new formulation of glucocerebrosidase prepared from human fibroblasts, for treatment of the nonneurologic form of Gaucher’s disease (Type 1). Patients with Gaucher’s disease have a congenital deficiency of glucocerebrosidase that leads to accumulation of glucosylceramide, the end-product of sphingolipid catabolism, in the lysozymes of reticuloendothelial cells in the liver, spleen and bone marrow.Velaglucerase is the second form of the enzyme now available in the US; imiglucerase (Cerezyme – Genzyme), which is produced by recombinant DNA technology from Chinese hamster ovary cells, was marketed earlier but has recently been in short supply.1 These agents are usually given as an IV infusion every 2 weeks. Both velaglucerase and imiglucerase have been shown to increase serum hemoglobin concentrations and platelet counts and decrease the size of the spleen. Velaglucerase contains the exact amino acid sequence of the human enzyme, while imiglucerase has one amino acid difference, but their activities are similar.2 The main difference between them may be in their cost. Cerezyme costs about $200,000 per year, while Vpriv is expected to cost about 15% less.2
1. Differentiation will be key challenge for Shire’s Gaucher disease drug Vpriv. The Pink Sheet. March 8, 2010; 72: 27.
2. B Brumshtein et al. Characterization of gene-activated human acid-beta-glucosidase: crystal structure, glycan composition, and internalization into macrophages. Glycobiology 2010; 20:24.
Download: U.S. English
1. Differentiation will be key challenge for Shire’s Gaucher disease drug Vpriv. The Pink Sheet. March 8, 2010; 72: 27.
2. B Brumshtein et al. Characterization of gene-activated human acid-beta-glucosidase: crystal structure, glycan composition, and internalization into macrophages. Glycobiology 2010; 20:24.
Download: U.S. English
Dutasteride (Avodart) for Prevention of Prostate Cancer
The Medical Letter on Drugs and Therapeutics • April 19, 2010; (Issue 1336)
A study recently published in the New England Journal of Medicine concluded that dutasteride (Avodart –
GlaxoSmithKline), a 5α-reductase inhibitor marketed for treatment of symptomatic benign prostatic...
A study recently published in the New England Journal of Medicine concluded that dutasteride (Avodart –
GlaxoSmithKline), a 5α-reductase inhibitor marketed for treatment of symptomatic benign prostatic hyperplasia, reduced the risk of prostate cancer over a 4-year period.
Edluar - A New Sublingual Formulation of Zolpidem
The Medical Letter on Drugs and Therapeutics • April 19, 2010; (Issue 1336)
Edluar (Meda), a new sublingual tablet formulation of the benzodiazepine receptor agonist zolpidem
(Ambien, and others), has been approved by the FDA for treatment of...
Edluar (Meda), a new sublingual tablet formulation of the benzodiazepine receptor agonist zolpidem
(Ambien, and others), has been approved by the FDA for treatment of insomnia.
Fentanyl Buccal Soluble Film (Onsolis) for Breakthrough Cancer Pain
The Medical Letter on Drugs and Therapeutics • April 19, 2010; (Issue 1336)
Fentanyl buccal soluble film (Onsolis – Meda) has been approved by the FDA for treatment of breakthrough pain in adult cancer patients who are already taking and are tolerant to around-the-clock opioid...
Fentanyl buccal soluble film (Onsolis – Meda) has been approved by the FDA for treatment of breakthrough pain in adult cancer patients who are already taking and are tolerant to around-the-clock opioid therapy. It is designated as a Schedule II controlled substance Two other oral transmucosal formulations of fentanyl are already available for this indication.
L-Methylfolate (Deplin) for Depression and Schizophrenia
The Medical Letter on Drugs and Therapeutics • April 19, 2010; (Issue 1336)
L-methylfolate (Deplin — Pamlab) is a “medical food” marketed for adjunctive use in depression or schizophrenia in patients with suboptimal folate levels. It is available only by...
L-methylfolate (Deplin — Pamlab) is a “medical food” marketed for adjunctive use in depression or schizophrenia in patients with suboptimal folate levels. It is available only by prescription.
Correction: Ferumoxytol (Feraheme)
The Medical Letter on Drugs and Therapeutics • April 19, 2010; (Issue 1336)
In the Medical Letter article on Ferumoxytol (Feraheme) - A New Parenteral Iron Formulation (2010; 52:23), the last sentence of the Dosage, Administration and Cost paragraph should have listed the cost of 1...
In the Medical Letter article on Ferumoxytol (Feraheme) - A New Parenteral Iron Formulation (2010; 52:23), the last sentence of the Dosage, Administration and Cost paragraph should have listed the cost of 1 gram of sodium ferric gluconate (Ferrlecit) as about $600.
Liraglutide (Victoza) for Type 2 Diabetes
The Medical Letter on Drugs and Therapeutics • April 5, 2010; (Issue 1335)
Liraglutide (Victoza – Novo Nordisk), a glucagon-like peptide-1 (GLP-1) receptor agonist given by subcutaneous
injection, has been approved by the FDA for treatment of patients with type 2 diabetes. It can...
Liraglutide (Victoza – Novo Nordisk), a glucagon-like peptide-1 (GLP-1) receptor agonist given by subcutaneous
injection, has been approved by the FDA for treatment of patients with type 2 diabetes. It can be used alone or in addition to oral antidiabetic drugs such as metformin (Glucophage, and others) or glimepiride (Amaryl, and others). Liraglutide is not recommended for first-line therapy and is not approved for use with insulin.
Plerixafor (Mozobil)
The Medical Letter on Drugs and Therapeutics • April 5, 2010; (Issue 1335)
The FDA has approved plerixafor (Mozobil – Genzyme), a CXCR4 chemokine receptor antagonist, for use in combination with granulocyte-colony stimulating factor (G-CSF; Neupogen) to mobilize peripheral blood...
The FDA has approved plerixafor (Mozobil – Genzyme), a CXCR4 chemokine receptor antagonist, for use in combination with granulocyte-colony stimulating factor (G-CSF; Neupogen) to mobilize peripheral blood stem cells in adults with multiple myeloma or non-Hodgkin’s lymphoma before high-dose chemotherapy with autologous stem cell rescue.
In Brief: Stopping Long-Acting Beta-2 Agonists
The Medical Letter on Drugs and Therapeutics • March 22, 2010; (Issue 1334)
A little more than a year ago, The Medical Letter reported the results of an FDA meta-analysis which found that use of a long-acting beta-2 agonist (LABA) such as salmeterol (Severent) or formoterol (Foradil)...
A little more than a year ago, The Medical Letter reported the results of an FDA meta-analysis which found that use of a long-acting beta-2 agonist (LABA) such as salmeterol (Severent) or formoterol (Foradil) in patients with asthma was associated with an increased risk of a composite endpoint of asthma-related death, intubation or hospitalization; the highest risk was in children 4-11 years old.There was no significant increase in risk when a long-acting beta-2 agonist was used with an inhaled corticosteroid.The Medical Letter recommended that long-acting beta-2 agonists should not be used as monotherapy for asthma, especially in children, and that long-acting beta-2 agonists should be used for asthma only in combination with an inhaled corticosteroid, preferably in a fixed-dose combination in the same inhaler.1
Now the FDA has issued new Safe Use Requirements2 and labeling requirements for long-acting beta-2 agonists that include the following: “Stop use of the LABA, if possible, once asthma control is achieved and maintain the use of an asthma-controller medication such as an inhaled corticosteroid.”3
It has not been determined that patients taking a longacting beta-2 agonist in a fixed-dose combination with an inhaled corticosteroid have an increased risk of death or that stopping long-acting beta-2 agonists in such patients will improve long-term outcomes. A controlled clinical trial of these new requirements would be welcome.
1. Long-acting beta-2 agonists in asthma. Med Lett Drugs Ther 2009; 51:1.
2. www.fda.gov/safety/medwatch/default.htm
3. BA Chowdhury and G Dal Pan. The FDA and safe use of long-acting beta-agonists in the treatment of asthma. N Engl J Med 2010; Feb 24 (epub).
Download: U.S. English
Now the FDA has issued new Safe Use Requirements2 and labeling requirements for long-acting beta-2 agonists that include the following: “Stop use of the LABA, if possible, once asthma control is achieved and maintain the use of an asthma-controller medication such as an inhaled corticosteroid.”3
It has not been determined that patients taking a longacting beta-2 agonist in a fixed-dose combination with an inhaled corticosteroid have an increased risk of death or that stopping long-acting beta-2 agonists in such patients will improve long-term outcomes. A controlled clinical trial of these new requirements would be welcome.
1. Long-acting beta-2 agonists in asthma. Med Lett Drugs Ther 2009; 51:1.
2. www.fda.gov/safety/medwatch/default.htm
3. BA Chowdhury and G Dal Pan. The FDA and safe use of long-acting beta-agonists in the treatment of asthma. N Engl J Med 2010; Feb 24 (epub).
Download: U.S. English
A Morphine/Naltrexone Combination (Embeda) for Pain
The Medical Letter on Drugs and Therapeutics • March 22, 2010; (Issue 1334)
The FDA has approved an agonist/antagonist combination of morphine and naltrexone (Embeda – King)
for treatment of chronic moderate to severe pain requiring around-the-clock analgesia for an extended
period...
The FDA has approved an agonist/antagonist combination of morphine and naltrexone (Embeda – King)
for treatment of chronic moderate to severe pain requiring around-the-clock analgesia for an extended
period of time. The addition of naltrexone is intended to prevent abuse of morphine.
Ferumoxytol (Feraheme) - A New Parenteral Iron Formulation
The Medical Letter on Drugs and Therapeutics • March 22, 2010; (Issue 1334)
Ferumoxytol (Fer yoo mox’ i tole; Feraheme – AMAG), an intravenous (IV) iron replacement product, has been approved by the FDA for treatment of iron deficiency anemia in adults with chronic kidney disease....
Ferumoxytol (Fer yoo mox’ i tole; Feraheme – AMAG), an intravenous (IV) iron replacement product, has been approved by the FDA for treatment of iron deficiency anemia in adults with chronic kidney disease. Iron deficiency anemia is common in chronic kidney disease and may be associated with decreased absorption from the gastrointestinal tract, limiting the usefulness of oral iron replacement. IV iron replacement can lower the dose requirement for erythropoiesis-stimulating drugs, particularly in patients on dialysis
Combination Oral Contraceptives and the Risk of Venous Thromboembolism
The Medical Letter on Drugs and Therapeutics • March 22, 2010; (Issue 1334)
Combination oral contraceptives increase the risk of venous thromboembolism (VTE). Their benefits, in
addition to preventing pregnancy, include lowering the risk of ovarian and endometrial cancer, reducing...
Combination oral contraceptives increase the risk of venous thromboembolism (VTE). Their benefits, in
addition to preventing pregnancy, include lowering the risk of ovarian and endometrial cancer, reducing dysfunctional uterine bleeding and increasing serum hemoglobin concentrations. Are these benefits worth
the risk? And are some combination oral contraceptives safer than others?
Rosiglitazone (Avandia) Revisited
The Medical Letter on Drugs and Therapeutics • March 8, 2010; (Issue 1333)
The cardiovascular safety of the thiazolidinedione rosiglitazone (Avandia – GlaxoSmithKline) is in the news again, with some authorities calling for its removal from the market (New York Times, February 19,...
The cardiovascular safety of the thiazolidinedione rosiglitazone (Avandia – GlaxoSmithKline) is in the news again, with some authorities calling for its removal from the market (New York Times, February 19, 2010).
Hyperbaric Oxygen Therapy for Refractory Wounds
The Medical Letter on Drugs and Therapeutics • March 8, 2010; (Issue 1333)
Hyperbaric oxygen (HBO2) therapy, breathing 100% O2 while exposed to increased atmospheric pressure, has been used for years to treat refractory wounds, especially diabetic foot...
Hyperbaric oxygen (HBO2) therapy, breathing 100% O2 while exposed to increased atmospheric pressure, has been used for years to treat refractory wounds, especially diabetic foot ulcers.
Primary Prevention of Ulcers in Patients Taking Aspirin or NSAIDs
The Medical Letter on Drugs and Therapeutics • March 8, 2010; (Issue 1333)
Aspirin and other nonsteroidal anti-inflammatory drugs (NSAIDs) are common causes of peptic ulcer disease. Patients infected with Helicobacter pylori who take aspirin or another NSAID have an especially high...
Aspirin and other nonsteroidal anti-inflammatory drugs (NSAIDs) are common causes of peptic ulcer disease. Patients infected with Helicobacter pylori who take aspirin or another NSAID have an especially high risk. Drugs that have been tried for prevention of ulcers in patients taking NSAIDs including H2-receptor antagonists, proton pump inhibitors (PPIs), aluminum- or magnesium-containing antacids, the prostaglandin misoprostol (Cytotec, and others), and antibiotics to eradicate H. pylori.
Click here to view the free full article.
Click here to view the free full article.
Addendum: Cost of Ustekinumab (Stelara)
The Medical Letter on Drugs and Therapeutics • March 8, 2010; (Issue 1333)
In the Medical Letter article on ustekinumab (Stelara) for psoriasis (2010; 52:7), footnote 2 in table 2 should have included a second sentence: $5595.60 is the cost of one 45-mg...
In the Medical Letter article on ustekinumab (Stelara) for psoriasis (2010; 52:7), footnote 2 in table 2 should have included a second sentence: $5595.60 is the cost of one 45-mg syringe.
Topical Oxybutynin (Gelnique) for Overactive Bladder
The Medical Letter on Drugs and Therapeutics • February 8, 2010; (Issue 1331)
The FDA has approved the marketing of a 10% topical gel formulation of the muscarinic receptor antagonist oxybutynin chloride (Gelnique - Watson) for treatment of overactive bladder. Oxybutynin is also...
The FDA has approved the marketing of a 10% topical gel formulation of the muscarinic receptor antagonist oxybutynin chloride (Gelnique - Watson) for treatment of overactive bladder. Oxybutynin is also available for this indication as oral tablets, an oral syrup and a transdermal patch.
Bepotastine (Bepreve) - An Ophthalmic H1-Antihistamine
The Medical Letter on Drugs and Therapeutics • February 8, 2010; (Issue 1331)
Bepotastine besilate 1.5% ophthalmic solution (Bepreve - ISTA Pharmaceuticals), an H1-antihistamine, has been approved by the FDA for topical treatment of itching associated with allergic conjunctivitis in...
Bepotastine besilate 1.5% ophthalmic solution (Bepreve - ISTA Pharmaceuticals), an H1-antihistamine, has been approved by the FDA for topical treatment of itching associated with allergic conjunctivitis in patients ≥2 years old. Bepotastine was first developed in an oral systemic formulation and has been used as such for treatment of allergic rhinitis in Japan.
Medical Marijuana
The Medical Letter on Drugs and Therapeutics • January 25, 2010; (Issue 1330)
Fourteen states in the US - Alaska, California, Colorado, Hawaii, Maine, Michigan, Montana, Nevada, New Jersey, New Mexico, Oregon, Rhode Island, Vermont and Washington - now permit, or soon will permit, some...
Fourteen states in the US - Alaska, California, Colorado, Hawaii, Maine, Michigan, Montana, Nevada, New Jersey, New Mexico, Oregon, Rhode Island, Vermont and Washington - now permit, or soon will permit, some medical use of marijuana (Cannabis sativa). In some states, licensed facilities dispense botanical cannabis by prescription. In others, limited self-cultivation is permitted for medical use.
Glucose Control in the ICU
The Medical Letter on Drugs and Therapeutics • January 25, 2010; (Issue 1330)
Once thought to be a beneficial response to critical illness, hyperglycemia is now recognized as independently associated with death and other adverse outcomes in various groups of critically ill patients....
Once thought to be a beneficial response to critical illness, hyperglycemia is now recognized as independently associated with death and other adverse outcomes in various groups of critically ill patients. Whether normalization of blood glucose by insulin infusion is beneficial in such patients has been a subject of debate in the critical care community. Some new guidelines have been published.
Click here to view the free full article.
Click here to view the free full article.
Ustekinumab (Stelara) for Psoriasis
The Medical Letter on Drugs and Therapeutics • January 25, 2010; (Issue 1330)
The FDA has approved the use of ustekinumab (Stelara - Centocor Ortho Biotech), an interleukin antagonist given by subcutaneous (SC) injection for treatment of adults with moderate to severe plaque psoriasis....
The FDA has approved the use of ustekinumab (Stelara - Centocor Ortho Biotech), an interleukin antagonist given by subcutaneous (SC) injection for treatment of adults with moderate to severe plaque psoriasis. It is the first agent in its class for this indication; the other biologic agents for psoriasis are Tcell or tumor necrosis factor (TNF) inhibitors.
A Third Amlodipine/ARB Combination (Twynsta) for Hypertension
The Medical Letter on Drugs and Therapeutics • January 11, 2010; (Issue 1329)
The FDA has approved Twynsta (Boehringer Ingelheim), a fixed-dose combination of the calcium-channel blocker (CCB) amlodipine and the angiotensin receptor blocker (ARB) telmisartan, for treatment of...
The FDA has approved Twynsta (Boehringer Ingelheim), a fixed-dose combination of the calcium-channel blocker (CCB) amlodipine and the angiotensin receptor blocker (ARB) telmisartan, for treatment of hypertension.
Intravenous Ibuprofen (Caldolor)
The Medical Letter on Drugs and Therapeutics • January 11, 2010; (Issue 1329)
An intraveneous (IV) formulation of ibuprofen (Caldolor - Cumberland) was recently approved by the FDA for use in adults. It can be administered alone for treatment of mild to moderate pain or as an adjunct to...
An intraveneous (IV) formulation of ibuprofen (Caldolor - Cumberland) was recently approved by the FDA for use in adults. It can be administered alone for treatment of mild to moderate pain or as an adjunct to opioid analgesics for moderate to severe pain. It is also approved for reduction of fever.
