ISSUE1675
- Mark Abramowicz, M.D., President has disclosed no relevant financial relationships.
- Jean-Marie Pflomm, Pharm.D., Editor in Chief has disclosed no relevant financial relationships.
- Brinda M. Shah, Pharm.D., Consulting Editor has disclosed no relevant financial relationships.
- Michael Viscusi, Pharm.D., Associate Editor has disclosed no relevant financial relationships.
- Discuss the approval of over-the-counter sale of naloxone 4-mg nasal spray (Narcan) for treatment of opioid overdose.
The FDA has approved the over-the-counter (OTC) sale of Narcan (Emergent), a nasal spray that delivers 4 mg of the opioid antagonist naloxone. Narcan nasal spray has been available by prescription since 2015 for emergency treatment of opioid overdose. Generic formulations of Narcan have also been approved; the manufacturers of these products will be required to switch them to OTC status and amend their labeling accordingly.1 Kloxxado, an 8-mg naloxone nasal spray, remains available only by prescription.2
Naloxone is the drug of choice for reversal of opioid overdose. Approval of the OTC sale of Narcan was based on data demonstrating that the drug is safe and effective for use as directed in the proposed labeling and that consumers can use it properly without the supervision of a healthcare professional.1
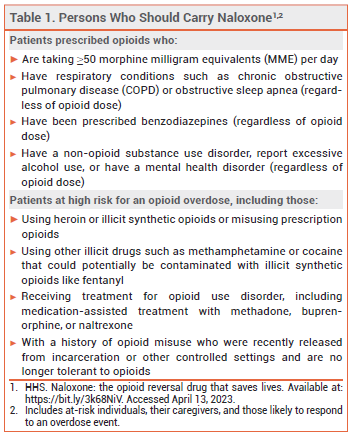
Every state in the US now has a naloxone access law; in most states, these laws grant both civil and criminal immunity to laypersons who administer naloxone.3 The US Department of Health and Human Services has recommended that certain individuals who are prescribed opioids or are at high risk for an opioid overdose, their caregivers, and persons who are likely to respond to an overdose event carry naloxone nasal spray (see Table 1).4
Narcan is not yet available OTC; announcements about its OTC availability and cost are expected in the coming months.1
- FDA News Release. FDA approves first over-the-counter naloxone nasal spray. March 29, 2023. Available at: http://bit.ly/3mek5Xy. Accessed April 13, 2023.
- In brief: Higher-dose naloxone nasal spray (Kloxxado) for opioid overdose. Med Lett Drugs Ther 2021; 63:151.
- Legislative Analysis and Public Policy Association. Naloxone: summary of state laws. July 2022. Available at: https://bit.ly/3ZNK1H9. Accessed April 13, 2023.
- HHS. Naloxone: the opioid reversal drug that saves lives. Available at: https://bit.ly/3k68NiV. Accessed April 13, 2023.