ISSUE1739
- Mark Abramowicz, M.D., President has disclosed no relevant financial relationships.
- Jean-Marie Pflomm, Pharm.D., Editor in Chief has disclosed no relevant financial relationships.
- Amy Faucard, MLS, Associate Editor has disclosed no relevant financial relationships.
- Discuss the efficacy of pramipexole for treatment-resistant depression.
The oral nonergot dopamine agonist pramipexole (Mirapex ER, and generics), which is FDA-approved for treatment of Parkinson's disease and restless legs syndrome, has been used off-label in patients with treatment-resistant depression (TRD).1 A double-blind, placebo-controlled trial of pramipexole augmentation in patients with unipolar TRD was recently published.2
TREATMENT OF TRD — When antidepressant monotherapy is inadequate, augmentation with a second-generation antipsychotic drug such as aripiprazole (Abilify, and others) is often effective, but these drugs can cause weight gain, metabolic adverse effects, and extrapyramidal symptoms.3 Addition of a second antidepressant from a different class is an alternative. Ketamine is increasingly being used off-label in patients with TRD; it can rapidly, though transiently, improve symptoms of depression. Esketamine, the S-enantiomer of ketamine, is FDA-approved as a clinician-administered nasal spray (Spravato) for use in adults with TRD. Other options include electroconvulsive therapy and off-label use of low-dose lithium, thyroid hormone, or, in patients with persistent anxiety, buspirone.4
PRAMIPEXOLE — Pramipexole is a full dopamine agonist with a high affinity for D3 receptors, which are associated with regulation of motivation and reward.5
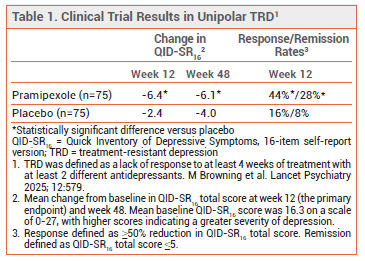
CLINICAL STUDIES — The unipolar TRD trial included 150 adults who were randomized to receive pramipexole or placebo for 48 weeks in addition to their current antidepressant treatment. Use of antipsychotic drugs was not allowed. Patients received immediate-release pramipexole once nightly at a starting dose of 0.25 mg. The dose could be increased every 3 days over a period of 4 weeks to a target dose of 2.5 mg if tolerated. The mean dose at 12 weeks was 2.3 mg. Pramipexole significantly reduced symptoms of depression compared to placebo at 12 and 48 weeks (see Table 1).2
In a comparative observational study in 132 patients with unipolar TRD, pramipexole or aripiprazole was added to the current antidepressant regimen; response and remission rates were higher with pramipexole (median maximum dose 1.05 mg/day) than with aripiprazole (median maximum dose 3 mg/day) at 12 and 24 weeks.6
Data from observational studies suggest that pramipexole can be effective in patients with bipolar depression,7 but a recently published double-blind trial in 39 adults with bipolar TRD found that addition of pramipexole (max 2.5 mg/day) to standard mood-stabilizing treatment did not significantly reduce depressive symptoms at 12 weeks compared to placebo.5 Larger controlled trials of pramipexole use in bipolar TRD are needed.
ADVERSE EFFECTS — Common adverse effects of pramipexole in the unipolar TRD trial included nausea, headache, dizziness, and sleep disturbance or somnolence. Treatment discontinuation due to adverse effects was more frequent with pramipexole than with placebo (20% vs 5%). Slowing the titration of the dose may improve tolerability.
Pramipexole can cause peripheral dopaminergic effects such as lower-extremity edema, nausea, vomiting, and orthostatic hypotension. Confusion and psychosis can occur, particularly in elderly patients. Impulse control disorders such as pathologic gambling, hypersexuality, uncontrollable spending, and excessive computer use are common in patients taking a dopamine agonist for Parkinson’s disease; two patients taking pramipexole in the unipolar TRD trial developed impulse control difficulties that resolved when the dose was reduced.8
DRUG INTERACTIONS — Most antipsychotic drugs are dopamine antagonists and could reduce the effectiveness of pramipexole.
COST — The wholesale acquisition cost for a 30-day supply of generic immediate-release pramipexole at a dose of 2.5 mg/day is about $13.9
CONCLUSION — In a 48-week, double-blind trial in patients with unipolar treatment-resistant depression, adjunctive treatment with the dopamine agonist pramipexole (not an FDA-approved use) significantly reduced depressive symptoms compared to placebo, but discontinuation of the drug due to intolerance was common.
- A Tundo et al. Pramipexole augmentation for treatment-resistant unipolar and bipolar depression in the real world: a systematic review and meta-analysis. Life (Basel) 2023; 13:1043. doi:10.3390/life13041043
- M Browning et al. Pramipexole augmentation for the acute phase of treatment-resistant, unipolar depression: a placebo-controlled, double-blind, randomised trial in the UK. Lancet Psychiatry 2025; 12:579. doi:10.1016/s2215-0366(25)00194-4
- Cariprazine (Vraylar) for adjunctive treatment of depression. Med Lett Drugs Ther 2023; 65:84.
- Drugs for depression. Med Lett Drugs Ther 2023; 65:193.
- H McAllister-Williams. Pramipexole in addition to mood stabilisers for treatment-resistant bipolar depression: the PAX-BD randomised double-blind placebo-controlled trial. Health Technol Assess 2025; 29:1. doi:10.3310/hbfc1953
- A Tundo et al. Comparative short- and long-term effectiveness and safety of pramipexole and aripiprazole augmentation in treatment-resistant unipolar depression: an observational study. Biomedicines 2024; 12:2064. doi:10.3390/biomedicines12092064
- A Tundo et al. Pramipexole in the treatment of unipolar and bipolar depression. A systematic review and meta-analysis. Acta Psychiatr Scand 2019; 140:116. doi:10.1111/acps.13055
- Drugs for Parkinson's disease. Med Lett Drugs Ther 2021; 63:25.
- Approximate WAC. WAC = wholesaler acquisition cost or manufacturer's published price to wholesalers; WAC represents a published catalogue or list price and may not represent an actual transactional price. Source: AnalySource® Monthly. September 5, 2025. Reprinted with permission by First Databank, Inc. All rights reserved. ©2025. www.fdbhealth.com/drug-pricing-policy.