ISSUE1740
- Mark Abramowicz, M.D., President has disclosed no relevant financial relationships.
- Jean-Marie Pflomm, Pharm.D., Editor in Chief has disclosed no relevant financial relationships.
- Susan Daron, Pharm D., Associate Editor has disclosed no relevant financial relationships.
- Discuss the 2025-2026 recommendations for treatment and prophylaxis of seasonal influenza.
- Compare the antiviral drugs available for treatment of influenza based on their efficacy, dosage and administration, and potential adverse effects.
- Determine the most appropriate regimen for treatment or prophylaxis of influenza in a particular patient.
- Influenza antiviral drugs recommended for use this season include three neuraminidase inhibitors (oral oseltamivir, IV peramivir, and inhaled zanamivir) and the oral cap-dependent endonuclease inhibitor baloxavir marboxil. They are all active against influenza A and B viruses.
- Antiviral treatment is most effective when started within 48 hours after illness onset.
- Antiviral treatment is recommended as soon as possible for patients with suspected or confirmed influenza who are hospitalized, have severe, complicated, or progressive illness, or are at increased risk for complications, even if it is started more than 48 hours after illness onset.
- Antiviral treatment can be considered for otherwise healthy symptomatic outpatients with suspected or confirmed influenza who are not at increased risk for influenza complications if it can be started within 48 hours after illness onset.
- Oseltamivir is preferred for treatment of children, pregnant women, hospitalized patients, and outpatients with severe, complicated, or progressive illness.
- Post-exposure prophylaxis with oseltamivir, zanamivir, or baloxavir should be considered within 48 hours of exposure for persons at high risk of complications who have not received an annual influenza vaccine or when influenza vaccination may be ineffective; it is not recommended for healthy persons exposed to influenza.
- Post-exposure prophylaxis with oseltamivir or zanamivir is recommended to control influenza outbreaks in institutional settings.
Tables
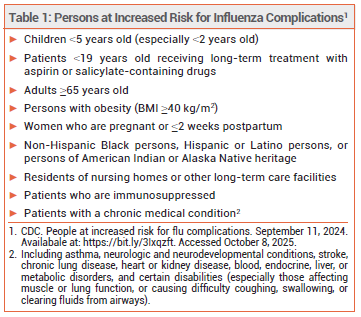
Influenza is generally a self-limited illness, but complications including pneumonia, respiratory failure, and death can occur, especially in persons at increased risk (see Table 1).
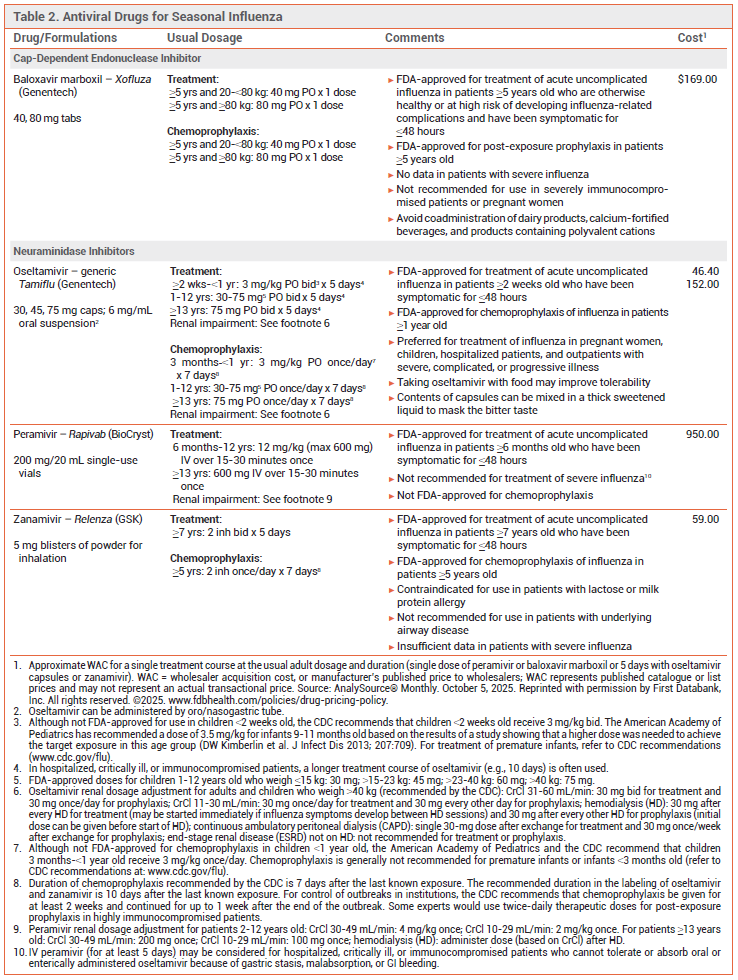
TREATMENT OF INFLUENZA — Three neuraminidase inhibitors (oral oseltamivir, IV peramivir, and inhaled zanamivir) and the oral cap-dependent endonuclease inhibitor baloxavir marboxil are available in the US and are recommended for treatment of influenza this season (see Table 2).1
Antiviral treatment is recommended as soon as possible for any patient with suspected or confirmed influenza who is hospitalized, has severe, complicated, or progressive illness, or is at increased risk for complications (see Table 1), even if it is started >48 hours after illness onset.1-3 False-negative results can occur with influenza tests, especially with rapid antigen tests; patients with suspected influenza in the aforementioned groups should receive antiviral treatment even if they test negative, especially when influenza viruses are known to be circulating in the community.4
Antiviral treatment can be considered for otherwise healthy symptomatic outpatients with suspected or confirmed influenza who are not at increased risk for influenza complications if it can be started within 48 hours after illness onset.
Oseltamivir is preferred for treatment of influenza in pregnant women, children, hospitalized patients, and outpatients with severe, complicated, or progressive illness.1,3
Guidelines for treatment of community-acquired pneumonia (CAP) recommend antiviral treatment for patients with CAP who test positive for influenza regardless of the duration of illness before diagnosis.5
Effectiveness – Neuraminidase inhibitors and baloxavir are active against influenza A and B viruses. In adults with acute uncomplicated influenza, they shorten the duration of symptoms by about one day.6-8 Although most controlled trials of antiviral drugs have not been powered to assess their efficacy in preventing serious influenza complications, experts have generally concluded from the combined results of observational studies, controlled trials, and meta-analyses that early antiviral treatment of influenza in high-risk and hospitalized patients can reduce the risk of complications.6,9-11
Outpatients – In a randomized, double-blind trial (CAPSTONE-2) in 2184 high-risk outpatients ≥12 years old with uncomplicated influenza, the median time to symptom improvement was similar with a single dose of baloxavir or 5 days’ treatment with oseltamivir (both started within 48 hours after illness onset) in the overall population and in those infected with influenza A(H3N2) virus, but was statistically significantly shorter with baloxavir in those infected with influenza B virus (74.6 vs 101.6 hours). Use of either drug was associated with a lower incidence of influenza-related complications and fewer antibiotic prescriptions compared to placebo.8
A meta-analysis of 73 randomized trials in patients with nonsevere influenza found that symptom duration was reduced by a mean of 1.02 days with baloxavir and 0.75 days with oseltamivir compared to placebo or standard care; baloxavir (but not oseltamivir) was associated with a possible reduction in the risk of hospitalization in high-risk patients.12
In a randomized, double-blind trial (miniSTONE-2) in 173 otherwise healthy children 1-11 years old with influenza, the median time to symptom improvement was similar with a single dose of baloxavir or 5 days’ treatment with oseltamivir (138 vs 150 hours; both started within 48 hours after illness onset).13
A meta-analysis of five randomized trials in children with influenza found that starting oseltamivir within 48 hours after illness onset reduced illness duration by about 18 hours (by about 30 hours when trials that enrolled children with asthma were excluded) and decreased the risk of otitis media.14
Hospitalized Patients – In a retrospective cohort study in 11,073 adults hospitalized for influenza (mean age 73 years), oseltamivir treatment was associated with a lower risk of in-hospital death compared to supportive care (adjusted risk difference -1.8%, 95% CI -2.8 to -0.9%).15
In a meta-analysis of six randomized controlled trials in hospitalized patients with severe influenza (mean age 36-60 years), treatment with oseltamivir or peramivir was associated with small reductions in the duration of hospitalization compared to placebo or standard care (-1.63 days with oseltamivir and -1.73 days with peramivir).16
In an observational cohort study in hospitalized children (with underlying conditions or admitted to the ICU) with laboratory-confirmed influenza, the duration of hospitalization was shorter with antiviral treatment started within 48 hours after illness onset than with no antiviral treatment.17
In a retrospective cohort study in 542 adults hospitalized with laboratory-confirmed influenza, time to defervescence, durations of hospital and ICU stay, and mortality rates were similar with oral oseltamivir and IV peramivir.18
In a randomized, double-blind trial (FLAGSTONE) in 366 patients ≥12 years old hospitalized with severe influenza requiring ventilation or supplemental oxygen or with an influenza-related complication, the combination of a neuraminidase inhibitor (primarily oseltamivir) and baloxavir did not significantly improve median time to clinical improvement compared to a neuraminidase inhibitor alone (97.5 vs 100.2 hours).19
Timing – Neuraminidase inhibitors are most effective when started within 48 hours after illness onset, but the results of some observational studies in hospitalized and critically ill patients suggest that treatment started as late as 4-5 days after illness onset can shorten the duration of hospitalization and reduce the risk of pneumonia, respiratory failure, and death.20-22
No data are available on the efficacy of baloxavir when started >48 hours after illness onset.
In a retrospective cohort study of 26,233 hospitalized adults with laboratory-confirmed influenza and pneumonia, delayed initiation of antiviral treatment was associated with a higher risk of death; compared to patients who started antiviral treatment on day 0, the adjusted odds ratio for death was 1.14 (95% CI 1.01-1.27) in those who started treatment on day 1 and 1.40 (95% CI 1.17-1.66) in those who started on days 2-5.23
In an observational study in 840 hospitalized adults with influenza, the risk of disease progression, ICU admission, and in-hospital death was lower with early use of oseltamivir compared to late use or no use of the drug.24
View the Comparison Table: Antiviral Drugs for Seasonal Influenza for 2025-2026
CHEMOPROPHYLAXIS — Oseltamivir, zanamivir, and baloxavir are FDA-approved for chemoprophylaxis of influenza. Post-exposure prophylaxis should be considered within 48 hours of exposure for persons at high risk of complications who have not received an influenza vaccine for the current season, received one within the previous 2 weeks, or might not respond to vaccination, or when the match between the vaccine and circulating strains is poor. Post-exposure prophylaxis may be considered for unvaccinated persons who are in close contact with persons at high risk for influenza complications who are unable to be otherwise protected. It is not recommended for healthy persons exposed to influenza or when >48 hours have elapsed since exposure.2,3
Post-exposure prophylaxis with oral oseltamivir or inhaled zanamivir is recommended by the CDC for control of institutional influenza outbreaks.1
Effectiveness – Oseltamivir, zanamivir, and baloxavir have generally been about 70-90% effective in preventing influenza caused by susceptible strains of influenza A or B viruses.1,25
A meta-analysis of 33 trials found that prompt post-exposure prophylaxis with oseltamivir, zanamivir, or baloxavir can reduce the risk of symptomatic influenza in patients at high risk for severe disease.26
In a randomized, placebo-controlled trial, administration of a single dose of baloxavir to an index patient within 48 hours of symptom onset significantly reduced the incidence of laboratory-confirmed influenza transmission to household contacts (adjusted relative risk reduction 29%); transmission that resulted in symptoms in household contacts was not significantly lower with baloxavir (incidence 5.8% vs 7.6% with placebo).27
Timing – Prophylaxis with oseltamivir or zanamivir should be started no later than 48 hours after exposure and continued for 7 days after the last known exposure. A single dose of baloxavir taken within 48 hours of exposure is also an option.
For controlling institutional influenza outbreaks, the CDC recommends post-exposure prophylaxis with oral oseltamivir or inhaled zanamivir for at least 2 weeks; prophylaxis should be continued for up to 1 week after the end of the outbreak.
PREGNANCY AND LACTATION — Pregnant and postpartum women are at increased risk for severe complications of influenza. Empiric antiviral treatment should be started as soon as possible in those with suspected or confirmed influenza. Oseltamivir is preferred for treatment of pregnant or breastfeeding women. Zanamivir appears to also be safe for use during pregnancy. Data are insufficient on use of peramivir. Baloxavir is not recommended for treatment of pregnant or breastfeeding women because of a lack of data.28-32
Antiviral post-exposure prophylaxis can be considered for pregnant women and those who are ≤2 weeks postpartum who cannot receive an influenza vaccine or have severe immunodeficiencies or other medical conditions that make them unlikely to respond to influenza vaccination. Oseltamivir is preferred.30,31
RESISTANCE — Over 99% of recently circulating influenza virus strains tested by the World Health Organization have been susceptible to neuraminidase inhibitors and baloxavir.33 Reduced susceptibility of some influenza virus strains, particularly influenza A(H1N1) viruses, to oseltamivir or peramivir can emerge during or after treatment, especially in young children and immunocompromised patients with prolonged viral shedding.34-39 Resistant isolates have usually remained susceptible to zanamivir, but reduced susceptibility to the drug has been reported.40 In immunocompromised patients, a double dose of oseltamivir reduced the incidence of oseltamivir resistance compared to standard dosing, but it did not improve efficacy and caused more adverse effects.41
Amino acid substitutions associated with reduced susceptibility to baloxavir have occurred following treatment with a single dose of the drug.7,27,42 Reduced susceptibility to baloxavir appears to be more frequent in persons infected with influenza A(H3N2) or A(H1N1)pdm09 viruses, particularly children.33,43,44 Baloxavir monotherapy is not recommended for use in severely immunocompromised patients because of concerns that prolonged viral replication in such patients could lead to emergence of resistance.1 Oseltamivir and peramivir may be active against influenza virus strains with reduced susceptibility to baloxavir.45 Baloxavir is active against neuraminidase inhibitor-resistant strains of influenza A and B viruses, including A(H1N1), A(H5N1), A(H3N2), and A(H7N9).
The adamantanes amantadine and rimantadine are active against influenza A, but not influenza B, viruses. Resistance to these drugs has been reported in recent years and remains high (>99%) among circulating influenza A(H3N2) and A(H1N1)pdm09 viruses; neither amantadine nor rimantadine is recommended for treatment or chemoprophylaxis of influenza this season.
ADVERSE EFFECTS — Nausea, vomiting, and headache are the most common adverse effects of oseltamivir; taking the drug with food may minimize GI adverse effects. Oseltamivir has been associated with bradycardia in critically ill patients.46 Diarrhea, nausea, sinusitis, fever, and arthralgia have been reported with zanamivir. Inhalation of zanamivir can cause bronchospasm; the drug should not be used in patients with underlying airway disease. Diarrhea and neutropenia have occurred with peramivir.47 Baloxavir appears to cause less nausea and vomiting than oseltamivir.48
Hypersensitivity reactions, including anaphylaxis, have been reported with all of these drugs.
Neuropsychiatric events, including self-injury and delirium, have been reported in patients taking neuraminidase inhibitors or baloxavir, but a causal relationship has not been established, and neuropsychiatric dysfunction can be a complication of influenza itself. In a retrospective cohort study of children 5-17 years old enrolled in Tennessee Medicaid during the 2016-2017 and 2019-2020 influenza seasons, oseltamivir was associated with lower rates of neuropsychiatric events than untreated influenza (34.8% vs 59.9%; adjusted risk ratio 0.53, 95% CI 0.33-0.88).49
DRUG INTERACTIONS — Coadministration of dairy products, beverages, antacids, laxatives, multivitamins, or other products containing polyvalent cations (e.g., calcium, aluminum, iron, magnesium, selenium, zinc) can reduce serum concentrations of baloxavir and should be avoided.
USE WITH THE LIVE-ATTENUATED VACCINE — Use of oseltamivir or zanamivir within 48 hours before, peramivir within 5 days before, or baloxavir within 17 days before administration of the live-attenuated intranasal influenza vaccine (FluMist, FluMist Home) could inhibit replication of the vaccine virus, reducing the vaccine’s effectiveness, and is not recommended.50 Persons who receive any of these antiviral drugs during these specified times or within 2 weeks after receiving the live-attenuated vaccine should be revaccinated with an inactivated or recombinant influenza vaccine.51
- CDC. Influenza antiviral medications: summary for clinicians. December 8, 2023. Available at: https://bit.ly/48KeAna. Accessed October 8, 2025.
- TM Uyeki et al. Clinical practice guidelines by the Infectious Diseases Society of America: 2018 update on diagnosis, treatment, chemoprophylaxis, and institutional outbreak management of seasonal influenza. Clin Infect Dis 2019; 68:895. doi:10.1093/cid/ciy874
- Committee on Infectious Diseases. Recommendations for prevention and control of influenza in children, 2025-2026: policy statement. Pediatrics 2025 July 28 (epub). doi:10.1542/peds.2025-073620
- CDC. Information on interpretation of influenza testing results when virus is circulating for health care settings. August 31, 2020. Available at: https://bit.ly/3KKyWVs. Accessed October 8, 2025.
- JP Metlay et al. Diagnosis and treatment of adults with community-acquired pneumonia. An official clinical practice guideline of the American Thoracic Society and Infectious Diseases Society of America. Am J Respir Crit Care Med 2019; 200:e45. doi:10.1164/rccm.201908-1581st
- CC Butler et al. Oseltamivir plus usual care versus usual care for influenza-like illness in primary care: an open-label, pragmatic, randomised controlled trial. Lancet 2020; 395:42. doi:10.1016/s0140-6736(19)32982-4
- FG Hayden et al. Baloxavir marboxil for uncomplicated influenza in adults and adolescents. N Engl J Med 2018; 379:913. doi:10.1056/nejmoa1716197
- MG Ison et al. Early treatment with baloxavir marboxil in high-risk adolescent and adult outpatients with uncomplicated influenza (CAPSTONE-2): a randomised, placebo-controlled, phase 3 trial. Lancet Infect Dis 2020; 20:1204. doi:10.1016/s1473-3099(20)30004-9
- MK Doll et al. Safety and effectiveness of neuraminidase inhibitors for influenza treatment, prophylaxis, and outbreak control: a systematic review of systematic reviews and/or meta-analyses. J Antimicrob Chemother 2017; 72:2990. doi:10.1093/jac/dkx271
- J Katzen et al. Early oseltamivir after hospital admission is associated with shortened hospitalization: a 5-year analysis of oseltamivir timing and clinical outcomes. Clin Infect Dis 2019; 69:52. doi:10.1093/cid/ciy860
- Y Sharma et al. Effectiveness of oseltamivir in reducing 30-day readmissions and mortality among patients with severe seasonal influenza in Australian hospitalized patients. Int J Infect Dis 2021; 104:232. doi:10.1016/j.ijid.2021.01.011
- Y Gao et al. Antiviral medications for treatment of nonsevere influenza: a systematic review and network meta-analysis. JAMA Intern Med 2025; 185:293. doi:10.1001/jamainternmed.2024.7193
- J Baker et al. Baloxavir marboxil single-dose treatment in influenzainfected children: a randomized, double-blind, active controlled phase 3 safety and efficacy trial (miniSTONE-2). Pediatr Infect Dis J 2020; 39:700. doi:10.1097/inf.0000000000002747
- RE Malosh et al. Efficacy and safety of oseltamivir in children: systematic review and individual patient data meta-analysis of randomized controlled trials. Clin Infect Dis 2018; 66:1492. doi:10.1093/cid/cix1040
- AD Bai et al. Oseltamivir treatment vs supportive care for seasonal influenza requiring hospitalization. JAMA Netw Open 2025; 8:e2514508. doi:10.1001/jamanetworkopen.2025.14508
- Y Gao et al. Antivirals for treatment of severe influenza: a systematic review and network meta-analysis of randomised controlled trials. Lancet 2024; 404:753. doi:10.1016/s0140-6736(24)01307-2
- AP Campbell et al. Influenza antiviral treatment and length of stay. Pediatrics 2021; 148:e2021050417. doi:10.1542/peds.2021-050417
- JS Lee et al. Clinical effectiveness of intravenous peramivir versus oseltamivir for the treatment of influenza in hospitalized patients. Infect Drug Resist 2020; 13:1479. doi:10.2147/idr.s247421
- D Kumar et al. Combining baloxavir marboxil with standard-ofcare neuraminidase inhibitor in patients hospitalised with severe influenza (FLAGSTONE): a randomised, parallel-group, double-blind, placebo-controlled, superiority trial. Lancet Infect Dis 2022; 22:718. doi:10.1016/s1473-3099(21)00469-2
- JK Louie et al. Neuraminidase inhibitors for critically ill children with influenza. Pediatrics 2013; 132:e1539. doi:10.1542/peds.2013-2149
- SG Muthuri et al. Effectiveness of neuraminidase inhibitors in reducing mortality in patients admitted to hospital with influenza A H1N1pdm09 virus infection: a meta-analysis of individual participant data. Lancet Respir Med 2014; 2:395. doi:10.1016/s2213-2600(14)70041-4
- JK Louie et al. Treatment with neuraminidase inhibitors for critically ill patients with influenza A (H1N1)pdm09. Clin Infect Dis 2012; 55:1198. doi:10.1093/cid/cis636
- MW Tenforde et al. Timing of influenza antiviral therapy and risk of death in adults hospitalized with influenza-associated pneumonia, influenza hospitalization surveillance network (FluServ-NET), 2012-2019. Clin Infect Dis 2025; 80:461. doi:10.1093/cid/ciae427
- NM Lewis et al. Benefit of early oseltamivir therapy for adults hospitalized with influenza A: an observational study. Clin Infect Dis 2025; 81:190. doi:10.1093/cid/ciae584
- H Ikematsu et al. Baloxavir marboxil for prophylaxis against influenza in household contacts. N Engl J Med 2020; 383:309. doi:10.1056/nejmoa1915341
- Y Zhao et al. Antivirals for post-exposure prophylaxis of influenza: a systematic review and network meta-analysis. Lancet 2024; 404:764. doi:10.1016/s0140-6736(24)01357-6
- AS Monto et al. Efficacy of baloxavir treatment in preventing transmission of influenza. N Engl J Med 2025; 392:1582. doi:10.1056/nejmoa2413156
- EJ Chow et al. Clinical effectiveness and safety of antivirals for influenza in pregnancy. Open Forum Infect Dis 2021; 8:ofab138. doi:10.1093/ofid/ofab138
- V Ehrenstein et al. Oseltamivir in pregnancy and birth outcomes. BMC Infect Dis 2018; 18:519. doi:10.1186/s12879-018-3423-z
- American College of Obstetricians and Gynecologists. Influenza in pregnancy: prevention and treatment: ACOG Committee Statement No. 7. Obstet Gynecol 2024; 143:e24. doi:10.1097/aog.0000000000005479
- CDC. Recommendations for obstetric health care providers related to use of antiviral medications for the treatment and prevention of influenza. September 15, 2022. Available at: https://bit.ly/3O62Xxx. Accessed October 8, 2025.
- IK Oboho et al. Benefit of early initiation of influenza antiviral treatment to pregnant women hospitalized with laboratory-confirmed influenza. J Infect Dis 2016; 214:507. doi:10.1093/infdis/jiw033
- S Hussain et al. Global update on the susceptibilities of influenza viruses to neuraminidase inhibitors and the cap-dependent endonuclease inhibitor baloxavir, 2020-2023. Antiviral Res 2025; 241:106217. doi:10.1016/j.antiviral.2025.106217
- AC Hurt et al. Characteristics of a widespread community cluster of H275Y oseltamivir-resistant A(H1N1)pdm09 influenza in Australia. J Infect Dis 2012; 206:148. doi:10.1093/infdis/jis337
- C Renaud et al. H275Y mutant pandemic (H1N1) 2009 virus in immunocompromised patients. Emerg Infect Dis 2011; 17:653. doi:10.3201/eid1704.101429
- JW Tang et al. Transmitted and acquired oseltamivir resistance during the 2018-2019 influenza season. J Infect 2019; 79:612. doi:10.1016/j.jinf.2019.10.020
- R Roosenhoff et al. Viral kinetics and resistance development in children treated with neuraminidase inhibitors: the Influenza Resistance Information Study (IRIS). Clin Infect Dis 2020; 71:1186. doi:10.1093/cid/ciz939
- B Lina et al. Five years of monitoring for the emergence of oseltamivir resistance in patients with influenza A infections in the Influenza Resistance Information Study. Influenza Other Respir Viruses 2018; 12:267. doi:10.1111/irv.12534
- E Takashita et al. Influenza A(H1N1)pdm09 virus exhibiting enhanced cross-resistance to oseltamivir and peramivir due to a dual H275Y/G147R substitution, Japan, March 2016. Euro Surveill 2016; 21:pii=30258. doi:10.2807/1560-7917.es.2016.21.24.30258
- R Trebbien et al. Development of oseltamivir and zanamivir resistance in influenza A(H1N1)pdm09 virus, Denmark, 2014. Euro Surveill 2017; 22:30445. doi:10.2807/1560-7917.es.2017.22.3.30445
- E Mitha et al. Safety, resistance, and efficacy results from a phase IIIb study of conventional- and double-dose oseltamivir regimens for treatment of influenza in immunocompromised patients. Infect Dis Ther 2019; 8:613. doi:10.1007/s40121-019-00271-8
- T Uehara et al. Treatment-emergent influenza variant viruses with reduced baloxavir susceptibility: impact on clinical and virologic outcomes in uncomplicated influenza. J Infect Dis 2020; 221:346. doi:10.1093/infdis/jiz244
- E Takashita et al. Influenza A(H3N2) virus exhibiting reduced susceptibility to baloxavir due to a polymerase acidic subunit I38T substitution detected from a hospitalised child without prior baloxavir treatment, Japan, January 2019. Euro Surveill 2019; 24:1900170. doi:10.2807/1560-7917.es.2019.24.12.1900170
- E Takashita et al. Human-to-human transmission of influenza A(H3N2) virus with reduced susceptibility to baloxavir, Japan, February 2019. Emerg Infect Dis 2019; 25:2108. doi:10.3201/eid2511.190757
- M Seki et al. Adult influenza A (H3N2) with reduced susceptibility to baloxavir or peramivir cured after switching anti-influenza agents. IDCases 2019; 18:e00650. doi:10.1016/j.idcr.2019.e00650
- R MacLaren et al. Oseltamivir-associated bradycardia in critically ill patients. Ann Pharmacother 2021; 55:1318. doi:10.1177/1060028020988919
- Peramivir (Rapivab): an IV neuraminidase inhibitor for treatment of influenza. Med Lett Drugs Ther 2015; 57:17.
- Baloxavir marboxil (Xofluza) for treatment of influenza. Med Lett Drugs Ther 2018; 60:193.
- JW Antoon et al. Influenza with and without oseltamivir treatment and neuropsychiatric events among children and adolescents. JAMA Neurol 2025 August 4 (epub). doi:10.1001/jamaneurol.2025.1995
- Influenza vaccines for 2025-2026. Med Lett Drugs Ther 2025; 67:153.
- LA Grohskopf et al. Prevention and control of seasonal influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices – United States, 2025-26 influenza season. MMWR Morb Mortal Wkly Rep 2025;74:500. doi:10.15585/mmwr.mm7432a2