ISSUE1692
- Mark Abramowicz, M.D., President has disclosed no relevant financial relationships.
- Jean-Marie Pflomm, Pharm.D., Editor in Chief has disclosed no relevant financial relationships.
- Brinda M. Shah, Pharm.D., Consulting Editor has disclosed no relevant financial relationships.
- Review the three new extended-release injectable antipsychotic drugs and compare them based on their efficacy, dosage and administration, and adverse effects.
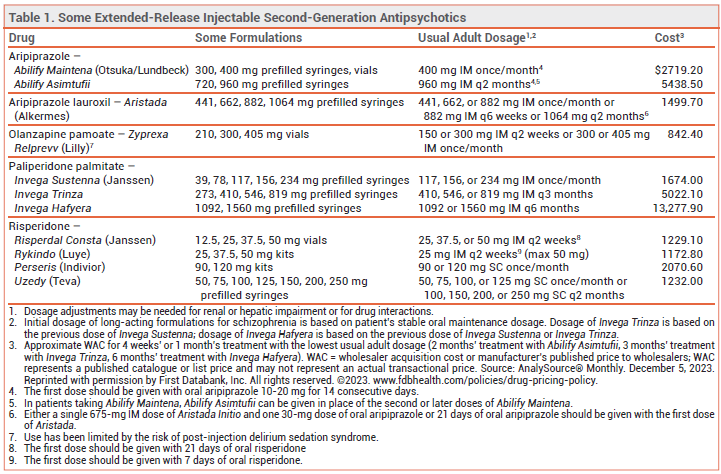
Three extended-release injectable formulations of second-generation antipsychotic drugs — two of risperidone (Rykindo, Uzedy) and one of aripiprazole (Abilify Asimtufii) — have been approved by the FDA for treatment of schizophrenia in adults. Rykindo and Abilify Asimtufii are also approved for maintenance treatment of bipolar I disorder in adults. Other extended-release injectable formulations of risperidone and aripiprazole have been available in the US for years (see Table 1).1

EXTENDED-RELEASE INJECTABLE ANTIPSYCHOTICS — Extended-release injectable second-generation antipsychotic drugs are generally used in patients with a history of relapse due to poor adherence to oral maintenance therapy. They are believed to improve adherence, hospitalization and relapse rates, and long-term outcomes in patients with schizophrenia, even though a benefit has not been consistently demonstrated in controlled trials comparing them with oral formulations.2-4
CLINICAL STUDIES — No new clinical efficacy trials were required for FDA approval of Rykindo or Abilify Asimtufii; approval was based on the results of earlier trials with other extended-release formulations of risperidone or aripiprazole. Approval of Uzedy was based on the results of earlier trials with oral risperidone and an unpublished trial (summarized in the package insert) in patients with schizophrenia. In the unpublished trial, patients received oral risperidone for 12 weeks, followed by either Uzedy or placebo given SC once monthly or once every 2 months. Time to relapse was statistically significantly longer with Uzedy than with placebo.
ADVERSE EFFECTS — The adverse effects of the new formulations of risperidone and aripiprazole appear to be similar to those observed with other injectable extended-release formulations of these drugs. Risperidone and aripiprazole can cause akathisia, angioedema, dyslipidemia, extrapyramidal symptoms, and hyperglycemia; risperidone can also cause hyperprolactinemia.
The labels of all second-generation antipsychotic drugs contain a boxed warning about an increased risk of death in older patients with dementia-related psychosis.
CONCLUSION — Extended-release injectable formulations of the second-generation antipsychotic drugs risperidone and aripiprazole have been available for years. The new extended-release formulation of aripiprazole (Abilify Asimtufii) and one new formulation of risperidone (Rykindo) do not appear to offer any advantage in efficacy and safety over other extended-release injectable formulations of these drugs. Uzedy, the other new extended-release formulation of risperidone, extends the interval between injections to once every 2 months, which is longer than with other extended-release injectable formulations of risperidone.
- Aripiprazole with digital ingestion tracking (Ability MyCite). Med Lett Drugs Ther 2019; 61:15.
- Drugs for psychotic disorders. Med Lett Drugs Ther 2016; 58:160.
- GA Keepers et al. The American Psychiatric Association practice guideline for the treatment of patients with schizophrenia. Am J Psychiatry 2020; 177:868. doi:10.1176/appi.ajp.2020.177901
- Drugs for bipolar disorder. Med Lett Drugs Ther 2016; 58:103.